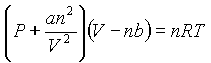
| MadSci Network: Chemistry |
While teaching the Van der Waal's equation for real gases, I came across something i did not completely understand: If the "b" constant is correlated to molecular volume, why would the b value for neon be smaller than the value for H or He? I checked the Handbook of Chemistry and Physics, and the closest I could come to an explanation was the fact that the b value was also correlated to a compressibility factor.
By the way, the van der Waals equation is
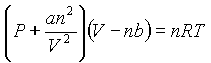
The first thing I did was to check my handy Sargent-Welch periodic table. It gives atomic and covalent radii. A few calculations (and a check of the Handbook of Chemistry and Physics) gave the following information:
| Element | Atomic Radius |
Molecular Volume |
van der Waals a constant* |
van der Waals b constant* |
| Hydrogen | 0.79 | 3.3 | 0.24 | 0.027 |
| Helium | 0.49 | 0.49 | 0.03 | 0.024 |
| Neon | 0.51 | 0.55 | 0.21 | 0.017 |
| * van der Waals constants taken from the Handbook of Chemistry and Physics, 61st Edition | ||||
For hydrogen (a diatomic molecule), we need the covalent radius (0.32) to convert the information into a molecular volume; I assumed a cylinder, with hemispheric ends, with radius 0.79 and length 2.22 (= 2*0.32+2*0.79). The volume is then given by:where rcov is the covalent radius of hydrogen. (The two hemispheres add to the volume of a sphere of radius r, and the remainder of the volume is a cylinder with radius r and height 2rcov.) For helium and neon, (monatomic) molecular volume is just
.
We must again be reminded that the van der Waals constants are empirical. Thus, they reflect many real-world variables, such as "compressibility." Compressibility ought to be affected by how well the molecules can interact with each other; the better the interactions, the higher the compressibility.
You see, the stronger the non-bonded intermolecular (that is, the van der Waals) forces, the more closely the molecules will be able to approach each other and the lower the value of the b constant. One source of van der Waals interactions is thought to be "induced-dipole/induced-dipole" interactions, in which a temporary dipole in one molecule induces an opposite dipole in a neighbor. The two temporary dipoles then attract each other.
However, the more tightly electrons are held within a molecule, the harder it will be to induce a dipole (this is called polarizability) and the weaker the van der Waals interactions. Indeed, the volume occupied by the molecules will go up because of electron-electron repulsions. Therefore, I think a clue to the van der Waals b constant may be found in ionization potentials, which measure how tightly electrons are held.
Looking at the three elements (and the same periodic table), we find:
| Element | Ionization Potential |
| Hydrogen | 13.6 |
| Helium | 24.6 |
| Neon | 21.6 |
I am afraid that I have been carried away!
| Dan Berger | |
| Bluffton College | |
| http://cs.bluffton.edu/~berger |
Try the links in the MadSci Library for more information on Chemistry.