![]()
| MadSci Network: Chemistry |
How does one produce H202 electrochemically? I know of the reaction K2SO4 + H20 gives K2SO3 + H202. But how is this implemented in practice? Does industry produce H202 in this way? Or are there other ways? I need this for a student's project: we're not going to actually produce it, but the student's need to be able to design a production process (a factory, that is) and think about the safety measures involved for peroxides and in electrochemistry. Would you also have some useful references for me, following the earlier discussion on how to make H2O2?
My source for this information is Chemistry of the Elements by N.N. Greenwood and A. Earnshaw, Pergamon Press (1984) -- a standard reference I recommend that every chemistry teacher buy!
At high current densities (with the associated safety problems), hydrogen peroxide may be produced by electrolytic oxidation of acidified sulfate solutions. The overall reaction is
![]()
Notice that the bisulfate is first oxidized to the peroxodisulfate, which hydrolyzes to yield H2O2 and regenerate the bisulfate. The net reaction is therefore
![]()
This process is only used in the laboratory nowadays, typically to produce perdeuterated hydrogen peroxide.
Industrial production uses a more complex and interesting process, which was developed by I.G. Farbenindustrie. 2-ethylanthroquinone is reduced (using Raney Nickel or a palladium catalyst) to the corresponding hydroquinone. The hydroquinone is then separated and oxidized using a stream of air, regenerating the quinone and converting dioxygen (O2) into hydrogen peroxide.
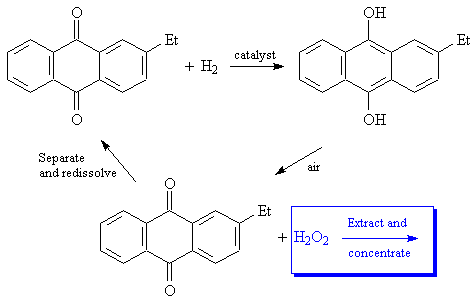
A reference for the industrial process: C.A. Crampton, G. Faber, J.P. Leaver and S. Schelle, "The manufacture, properties and uses of hydrogen peroxide and other inorganic peroxy compounds," in R. Thompson (ed.), The Modern Inorganic Chemicals Industry, pp. 232-66. Chemical Society Special Publications (RSC), No. 31, 1977.
| Dan Berger | |
| Bluffton College | |
| http://cs.bluffton.edu/~berger |
Try the links in the MadSci Library for more information on Chemistry.