| MadSci Network: Chemistry |
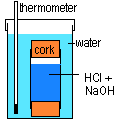
I assume you must be placing your tube in a beaker of water, and measuring the temperature change of the water. The best type of tubing to allow heat to escape would not be plastic at all, but metal. Copper tubing is probably the best and most easily avaiable.You can seal the bottom with a cork or rubber bung. Your tube should be completely immersed in the water, in which case you have to seal the top also. You should also have you water in an insulating container with a lid, to prevent heat loss. You would have to move quickly, sealing and immersing the copper tube as soon as you had added the second solution.

However, the best place to measure the temperature change would be inside the solutions themselves. The water in which the acid and alkali are dissolved is itself a kind of "surroundings" and absorbs the heat from the neutralisation. Measure the temperatures of the acid and alkali solutions before mixing (rinse the thermometer with water in between). If they are different, take an average (are you mixing equal volumes?). Then place your thermometer in one of the solutions, add the other, and observe the temperature. When it stops rising you have your final value. Again, use an insulating container with a lid, like a polystyrene (styrofoam) drinks cup.
Try the links in the MadSci Library for more information on Chemistry.