| MadSci Network: Physics |
Quantum Mechanics is a theory which says that when you make a measurement of a particle, there are only certain results you can get for an answer. An analogy would be the number of cents you have in your pocket. You can only have money in increments of one penny -- you can never have 23.14789 cents. Instead, the number of cents always has to be a round number.
This sounds natural for money -- after all, there's nothing smaller than a penny -- but it was a very surprising discovery in the early 1900's when it was found that physical measurements behave this way, too. For example, the electron in a hydrogen atom was found to exist only in certain energy levels -- or "quantum states". When an electron moves from one energy level to another, it doesn't gradually pass through all the energy levels inbetween. Instead, there is a "quantum leap", and the electron instantly leaps from one energy level to the next. In general, a quantum leap is when a particle changes from one quantum state to another.
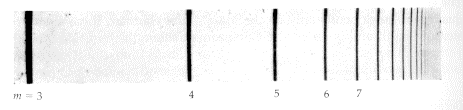
This particular example of the electron in a hydrogen atom can be measured by looking at the light given off when the electron "leaps" from one quantum state to another. If an electron drops to a lower energy state, conservation of energy demands that the extra energy has to go somewhere. In this case, the extra energy goes into a "photon", or a light particle. The frequency of the light tells how much energy is in each photon, which in turn tells us about what the electron is doing. Here is an image which shows the different frequencies of light that are emitted by Hydrogen atoms:

As you can see, the light only comes off in certain frequencies, which means that the electrons are only in certain energy states. This was one of the pieces of evidence found in the early 1900's which convinced physicists that nature really did have a "quantum" aspect.
The above image is taken from a web page which has much more information about the spacing of the lines and the quantum theory which explains it.
But is it still possible that the electron is actually moving through all the energy levels inbetween, but doing it too fast to see? Probably not, or the photons which are detected would have a different character -- they would not be a single particle but rather a bunch of different particles slightly spread out in time. Because the photons behave as single particles (you can't split them up into smaller sub-particles), it seems that the "quantum leap" really is happening.
As for "how it works", though... That's something only advanced physics, such as field theory, can attempt to explain -- and some people might claim we still don't understand it! If you're interested in learning more about how electrons and light might interact on a fundamental level (without also learning a bunch of painful math), try the book QED: The strange theory of light and matter by Richard Feynman.
One final amusing point: people often use the phrase "quantum leap" to talk about a huge advance in a technology or in a way of thinking. In fact, a quantum leap is the smallest change one can possibly make!
Try the links in the MadSci Library for more information on Physics.