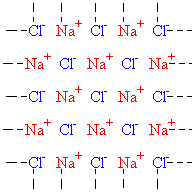
| MadSci Network: Chemistry |
Explain why fused or melted ionic compounds are good conductors of electricity and the solid crystals are not?
Electricity requires the movement of electrical charges. We normally think of moving electrons, but it is not necessary that the charges be electrons as long as they can move.
In an ionic crystal, all the particles making up the crystal are charged: for example, in sodium chloride there are positive sodium ions and negative chloride ions. In a sodium chloride crystal, the ions are stacked very much like oranges in a box: each orange fits into the gap between four oranges in the next layer down. A single layer of a sodium chloride crystal is represented below; notice that each row and column goes off to infinity! Also, above and below each sodium ion is a chloride ion in a neighboring layer; above and below each chloride ion is a sodium ion.
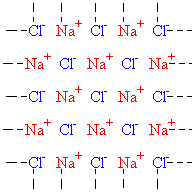
But suppose you tear open the crate and pour out the oranges. Now the oranges can move freely. This is similar to melting (fusing) an ionic compound. Since, in the liquid state (or the aqueous solution state, for that matter) ions are free to move about, fused or dissolved ionic compounds can conduct electricity.
| Dan Berger |
| Bluffton College |
| http://cs.bluffton.edu/~berger |
Try the links in the MadSci Library for more information on Chemistry.