| MadSci Network: Chemistry |
How can some aqueous ions be listed as having a negative entropy?
I thought that the smallest entropy assigned was zero (for a
perfect crystal at zero Kelvin). How can some aqueous ions be
assigned a negative value in thermodynamic tables?
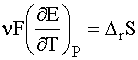
Furthermore, the standard partial molar entropy of the aquated proton is defined as zero:
Relative to this, many ions have negative solution entropies.
Now solution is very different from the solid state; we are really comparing apples to oranges here. Even so, we can make some hand-waving statements on the order of "well, the apple is red and the orange is orange, and they have different detailed shapes."
A perfect crystal is in a highly ordered state, but its component ions are not imposing order on their surroundings, so to speak. However, in solution the ions are solvated. They are therefore imposing a great deal of order on their surroundings, compared to the random arrangements of water molecules in pure water. You have gone from pure water plus a perfect crystal, to a series of ordered aggregates of the form ion· nH2O.
And remember that solvated H+ is assigned an entropy of zero. H+ is the smallest ion and so can't surround itself with as many water molecules as, say, Na+.
| Dan Berger | |
| Bluffton College | |
| http://cs.bluffton.edu/~berger |
Try the links in the MadSci Library for more information on Chemistry.