| MadSci Network: Chemistry |
Does the water's temperature affect how nucleophilic it is?
Is there a difference in nucleophility between hot and cold
water? If so, why? If not, why does butylchloride in cold water
result in substitution, while butylchloride in hot water results
in a certain degree of elimination as well (the higher the water-
temperature, the higher the degree of elimination)?
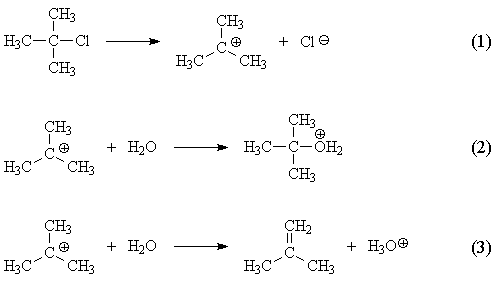
The answer to your question has nothing to do with nucleophilicity; nucleophilicity only comes into play when comparing different nucleophiles, for example, water with cyanide. Consider the mechanism of reaction of t-butyl chloride with water:

Steps (2) and (3) lead to different products, t-butyl alchohol and isobutene respectively. So we are dealing with a competition between (2) and (3) in regard to product formation.
While step (2) does not involve breaking any bonds, step (3) breaks a carbon-hydrogen bond. (Both (2) and (3) form bonds.) This would lead us to expect a less favorable enthalpy for step (3) than for step (2), and AM1 calculations bear this out.
More sophisticated readers of this answer should realize that we are implicitly considering terms in the activation free energy rather than in the overall free energy. I am using the somewhat crude approximation that there isn't much qualitative difference. Most of the arguments pertain to free energies of activation as well. The AM1 calculations were for transition state enthalpies.However, remember that we are ultimately concerned with free energy; the rate of reaction is inversely proportional to exp(DG). Since free energy is composed of both enthalpy and entropy,
we must also consider entropy. Notice that the entropy becomes more important as the temperature rises.
Since (2) goes from two molecules to one, and (3) goes from two molecules to two, (3) will have a more positive entropy than (2). Thus, as the temperature rises, elimination (3) should dominate over substitution (2).
At lower temperatures entropy is less important, and the fact that (2) has a lower enthalpy than (3) makes (2) happen faster at lower temperatures.
Again, a more sophisticated analysis might consider that at lower temperatures the reaction is irreversible and so is kinetically controlled (the barrier height is lower for substitution than for elimination). At higher temperatures, the more stable product would be expected to form (thermodynamic control), and the entropy boost for elimination would be the significant factor in making elimination the more stable product. (Substitution is estimated by AM1 and by bond enthalpies to be a more stable product than elimination. A rough estimate is that solution effects would cancel out.)
| Dan Berger | |
| Bluffton College | |
| http://cs.bluffton.edu/~berger |
Try the links in the MadSci Library for more information on Chemistry.