| MadSci Network: Chemistry |
Is there a trend in the P.T. for threshold frequency in photoelectric
effect?
There are trends for reactivity and so on, so is there an
obvious trend for the threshold frequency of the elements? Does
this trend have any bearing on the threshold frequency of
compounds formed by these elements? Lastly, what factors
influence the frequency?
F represents the energy required to knock an electron loose, and we would therefore expect any trend to correspond to electronegativities or ionization energies. Remember that E=hn, so that the lower the work function, the lower the frequency of light required to knock an electron loose.
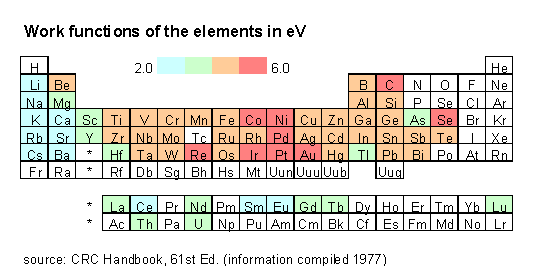
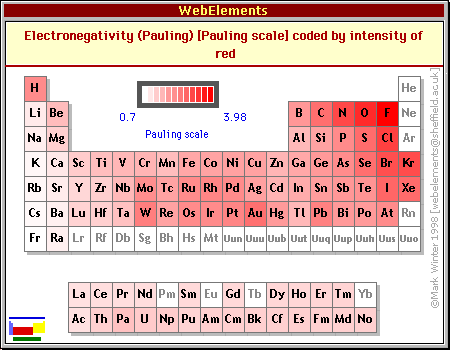
I have prepared a periodic table which shows F, in eV. Compare it with a table of electronegativities, taken from WebElements.


If you compare the two tables, you see that the value of F does tend to follow the Pauling electronegativity (and therefore the first ionization energy, which is pretty obvious). The correlation is not perfect; according to my CRC Handbook, not all the values for F are considered reliable. But the trend seems clear.
Does this trend have any bearing on the threshold frequency of compounds formed by these elements?
I was unable to find a table of values of F for compounds, but I expect that the trend does follow within very large error bars. Coordination compounds (such as Cu · 6H2O) have the electronic energy levels of the metal strongly influenced by the nature of the ligands. But I expect that the work functions of semiconducting compounds, such as GaAs or ZnS, should relate in some straightforward way to the values of F of the component elements. (A cursory glance at the table of semiconductor band-gaps in the CRC Handbook bears this out.)
Lastly, what factors influence the frequency?
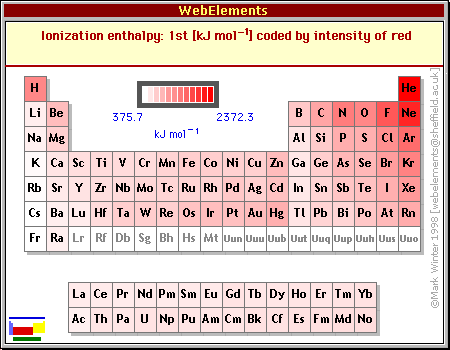
As I stated above, it seems intuitively obvious that the most important influence on the value of the work function should be the first ionization energy. Compare the two tables below (the table of ionization enthalpies is from WebElements):

For numerical values of the work function, click here.
| Dan Berger | |
| Bluffton College | |
| http://cs.bluffton.edu/~berger |
Try the links in the MadSci Library for more information on Chemistry.