| MadSci Network: Chemistry |
Because if p and d orbitals were circular, they'd be identical to the s orbitals!
A better answer would be that the s orbitals have zero orbital angular momentum, while the p orbitals have one quantum of orbital angular momentum which shapes the probability distribution differently. The mathematical functions which govern the shape of the orbital are called "spherical harmonics" -- you can think of them as different "modes", just as a vibrating string can have different harmonic modes depending on how fast it is vibrating.
As for why atoms are "happier" with filled orbitals... "Happy" is a simple way to talk about the lowest energy states. Without an outside energy source, electrons will decay away excess energy and drop into the lowest available energy state that they can find. They will stay in that state until an outside energy source comes along to knock them out. Because each orbital has a higher energy than the next, filling an orbital means that the electrons are compactly arranged into low energy states -- something that the atom is "happy" with.
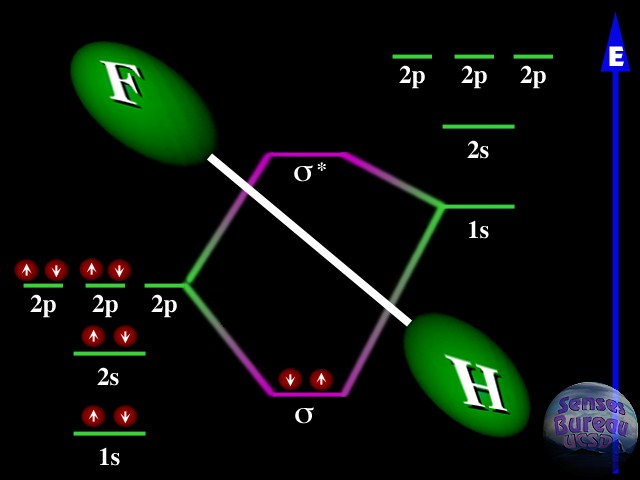
When two atoms with almost-filled orbitals come together, two empty atomic orbitals can merge into two empty molecular orbitals -- with a different energy structure. Here's a picture of a H-F bond:

You can see that the 2p state from the F atom merges with the 1s state from the H atom to form two "sigma" states. These states are molecular orbitals -- the electrons have a probability cloud around BOTH nuclei, not just around one. Because both H and F are "missing" one electron from these two states, one electron from each atom (the red circles with white spin- arrows) can fall into the lower-energy sigma state (the atoms are "happier" this way because it has less energy!). This bonds the two atoms together into a molecule.
For lots of great 3D pictures and movies which explain general chemistry, you absolutely have to check out this page put together by Kent Wilson's group at UCSD. (The above picture came from that website.) You'll find out lots more about different kinds of chemical bonds, pictures of molecular orbitals interacting, and even movies which show dynamic bonding!
Try the links in the MadSci Library for more information on Chemistry.