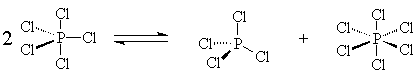
Solid "PCl5" is completely converted to the ionic form. See N.N. Greenwood and A. Earnshaw, Chemistry of the Elements, 1st Ed., Pergamon Press (1984), p. 573.
| Dan Berger |
| Bluffton College |
| http://cs.bluffton.edu/~berger |
| MadSci Network: Chemistry |
|
In the gas phase, PCl5 is a molecular
compound. But in condensed phases, it partially disproportionates into
PCl4+ and PCl6-. These ions are what allow liquid
"PCl5" to be an electrical
conductor.
Solid "PCl5" is completely converted to the ionic form. See N.N. Greenwood and A. Earnshaw, Chemistry of the Elements, 1st Ed., Pergamon Press (1984), p. 573.
|
Try the links in the MadSci Library for more information on Chemistry.