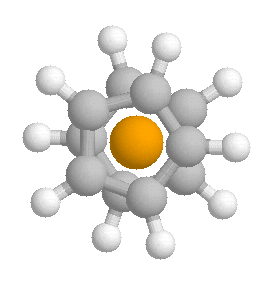
 The staggered structure of ferrocene
is an energy minimum; the all-eclipsed version is a transition state which
is not very much higher in energy than the minimum. The barrier for
rotation is, as you have said, only a few kJ/mol; what this means is that
at room temperature, in the gas phase and in solution, ferrocene's
cyclopentadienyl rings are spinning like tops. In the crystal, ferrocene exists as a staggered molecule; interactions
with neighboring molecules seem to damp down the spin enough for us to get
good X-ray fixes on the ring carbon atoms.
The staggered structure of ferrocene
is an energy minimum; the all-eclipsed version is a transition state which
is not very much higher in energy than the minimum. The barrier for
rotation is, as you have said, only a few kJ/mol; what this means is that
at room temperature, in the gas phase and in solution, ferrocene's
cyclopentadienyl rings are spinning like tops. In the crystal, ferrocene exists as a staggered molecule; interactions
with neighboring molecules seem to damp down the spin enough for us to get
good X-ray fixes on the ring carbon atoms.
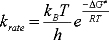 It's easy to estimate the rate for any reaction, for which the activation
energy is known or estimated. One just uses the pseudo-Arrhenius
relationship shown here (taken from Carey & Sundberg, Advanced
Organic Chemistry, Part A, p.191-192), and plug in the temperature
you're interested in. At 300 K and assuming 5 kJ/mol, I get a rate constant
of 8´1011 per second, or pretty
fast.
It's easy to estimate the rate for any reaction, for which the activation
energy is known or estimated. One just uses the pseudo-Arrhenius
relationship shown here (taken from Carey & Sundberg, Advanced
Organic Chemistry, Part A, p.191-192), and plug in the temperature
you're interested in. At 300 K and assuming 5 kJ/mol, I get a rate constant
of 8´1011 per second, or pretty
fast.
When I was in grad school I learned a rule of thumb that a reaction had to
have an activation energy of 20 kcal/mol or less (about 85 kJ/mol) to have
a reasonable rate at room temperature. For 85 kJ/mol I get a rate constant
of 10-2 per second.
|
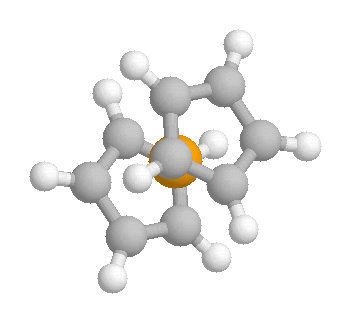 My textbook says that the rings in ferrorcene are constantly
rotating, but all of the sources I have looked at indicated that the rings
are staggered or eclipsed. How can they be constantly rotating if the
ring orientation can be known? Also, according to the references I have
found, ferrocene has an internal rotation barrier of 4-10kJ/mol. Is this
barrier just for the change of staggered to eclipsed or is it the barrier
for the rings to be constantly rotating?
My textbook says that the rings in ferrorcene are constantly
rotating, but all of the sources I have looked at indicated that the rings
are staggered or eclipsed. How can they be constantly rotating if the
ring orientation can be known? Also, according to the references I have
found, ferrocene has an internal rotation barrier of 4-10kJ/mol. Is this
barrier just for the change of staggered to eclipsed or is it the barrier
for the rings to be constantly rotating?
 The staggered structure of
The staggered structure of