NO.
If you look at a table of bond energies, you will find that covalent bonds between different kinds of atoms have different strengths. C-C single bonds are worth about 83 kcal/mol, while C-H bonds are worth about 99, and C-O about 86.
"But," you shout, "that's not what I mean at all! Both bonds are between two carbon atoms, so shouldn't they have the same strength!!!???"
NO.
The second bond in a carbon-carbon double bond is called a pi bond and is of a different type than the first bond, which is a sigma bond.
If you don't know the difference between a sigma bond and a pi bond, you should ask your chemistry teacher or read thi s MadSci answer.It turns out that, in ethylene and other compounds with carbon-carbon double bonds, it's pretty easy to estimate the strength of the pi bond:
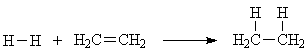
We know the deltaH for this reaction; it's -33 kcal/mol. We know the approximate bond energy of the single bonds involved: H-H is 104 kcal/mol, and C-H is 100 kcal/mol. So since we break one H-H bond as well as the pi bond, and form two C-H bonds, we can estimate the strength of the pi bond by
104 + x - 2*100 = -33
x = 2*100 - 104 - 33 = 63 kcal/mol
But there's more: you can have two C-C pi bonds at once, each with different strengths! Hydrogenation of acetylene to ethylene is also possible:
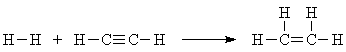
104 + y - 2*111 = -42 y = 2*111 - 104 - 42 = 76 kcal/mol.
So even two C-C pi bonds in the same molecule don't have the same strength! And in general we find that pi bond strength varies all over the map; for example, C-O sigma bonds are worth only about 86 kcal/mol, but the pi bond in a generic C=O is worth about 114 kcal/mol, and in carbon dioxide it goes up to about 140 kcal/mol!Of course it is a drastic oversimplification to pretend that bonds within the same molecule can be separated from each other! But that's the model we use, and it's very useful.But back to our specific case: you can polymerize ethylene exothermically, essentially because four C-C bonds are worth more than two C=C bonds. In other words, it costs you less to break the pi bond in ethylene than you get by forming a new sigma bond to the neighboring ethylene molecule, and so on and so forth on down the chain.
For more about polymers, go to the Macrogalleria.
| Dan Berger |
| Bluffton College |
| http://cs.bluffton.edu/~berger |