| MadSci Network: Chemistry |
My reference for this answer is the 4th Edition (1983) of that venerable organic chemistry text, Morrison and Boyd. However, you can find the same information in any college organic chemistry textbook.
Alkanes are non-polar molecules containing only carbon and hydrogen atoms, and only single bonds. Thus, the interaction between molecules are the relatively weak van der Waals forces, which increase as the surface area of the molecule increases.
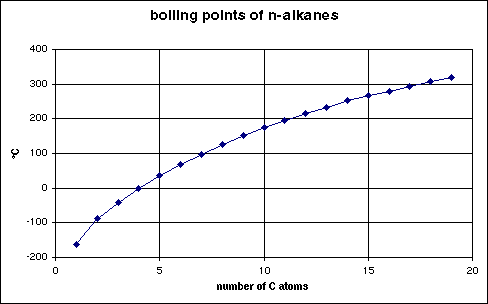
Boiling points depend almost exclusively upon the size of the molecule, thus, they rise fairly uniformly with the number of carbon atoms. (See chart below.)

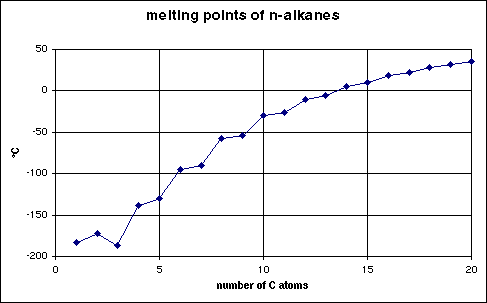
The intermolecular forces that must be overcome in melting depend not only upon size, but also upon how well the molecules fit into a regular crystal lattice. In general, highly symmetric and rigid molecules fit more easily into a regular crystal. (Think of the difference between rolled-up socks and loose socks in a dresser drawer!) This is most pronounced at the smallest carbon numbers. The chart below shows the relationship between carbon number and melting point. Notice how low the m.p. is for propane. Clearly propane does not fit a regular crystal very well.
If you make models of methane, ethane, and propane, you will notice that propane is "floppy", unlike methane and ethane. After 2 carbons, the carbon backbone of an alkane is not rigid and becomes more difficult to pack into a crystal.
Also notice that through undecane (11 carbons) the odd-numbered n-alkanes have slightly lower melting points than would be expected. I don't have a good explanation for this, but apparently odd-numbered carbon chains have more difficulty making a good crystal.
The story gets even more interesting when branched alkanes are considered.
Usually branching disrupts the crystal and decreases the melting point, but
if the molecule becomes very symmetric by virtue of branching, the melting
point rises as the symmetrical molecule fits nicely into a crystal lattice.
The best examples are the pentanes:
n-pentane, CH3CH2CH2CH2CH
3 has a m.p. of -130 C
isopentane, (CH3)2CHCH2CH3 has
a m.p. of -159 C,
neopentane, (CH3)4C has a m.p. of -17 C!
Try the links in the MadSci Library for more information on Chemistry.