Date: Mon Mar 6 13:12:50 2000
Posted By: Todd Holland, Grad student, Biophysics, University of Illinois at Urbana-Champaign
Area of science: Botany
ID: 951844826.Bt
Message:
Well, this may sound like a technicality, but in the strictest sense,
science cannot answer questions of why things are the way they are. It can
only tell us how they could have become that way
based on our knowledge of natural processes, such as evolution in the case
of biology. And even though we may think of organisms as being perfectly
crafted to fill their own particular
ecological niche, almost none of them truly are. One of my professors
compared the process of the evolution to trying to improve the design of
your car while you're driving it down the road. If
particularly bad design flaws are incorporated into a system that is vital
for your survival, you can't just scrap that system while you work on
developing something better. And also, because
natural selection, the driving force behind evolution, only occurs on
systems where improvements increase survival rates, an organism also can't
improve a new part without relying on it. So,
many biological systems have serious "design" flaws from an engineering
perspective. Photosynthesis is one of them. However, the major "flaw" in
photosynthesis is not in harvesting light.
Plants are actually very good at that. And the small window in the green
wavelengths where they don't absorb quite as well is actually fairly
shallow. Plants can absorb and use green light to do
photosynthesis, just not as well as they can red and blue light. Let's look
at some absorbance spectra, and I'll show you what I mean(1):
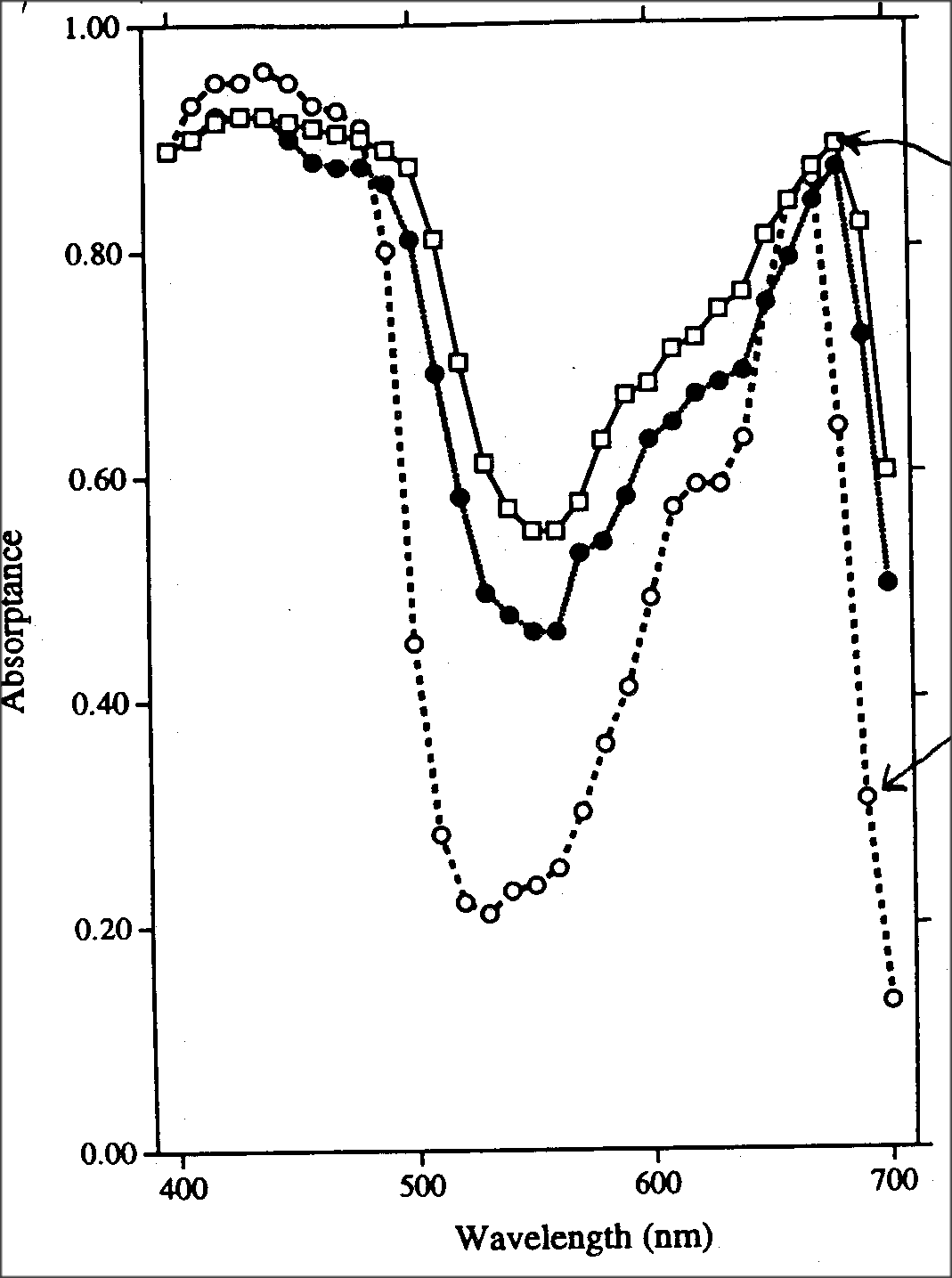 So in this experiment light of particular wavelengths was shined onto a few
materials, and then the amount that got through was measured. The x-axis is
the wavelength of light in nanometers
(10-9 meters), and the y-axis is the amount of light absorbed by the
material being observed. The absorbance is defined as A=log T0/T, where T
is the amount of light transmitted and T0 is the
initial amount of light before passing into the sample. An absorbance of
1.0 means that 90% of the light was absorbed, A=0.5 means about 2/3 was
absorbed, and A=0.0 means that it all got
through. There are two important traces here. One is at the top, and has
data points represented as open boxes. It is from a thin, single leaf. The
other important one is at the bottom and has
open circles. It is the absorbtion spectrum for chlorophyll that has been
extracted from a leaf. As you can see, although chlorophyll by itself
doesnít absorb very well in the green wavelengths
(the low trough on the graph), the whole leaf has A > 0.5, so it absorbs
over 2/3 of the incident light in the green wavelength. Also, we must
consider that most plants are part of a "canopy",
where light first encounters tall plants, and then what isnít absorbed by
the tallest plants goes down to the next level, and so on. So, eventually
almost all of even the green light is absorbed. The
wavelengths scanned here pretty much cover the visible spectrum.
Wavelengths shorter than this carry too much energy and can be actually
harmful, so the plant is better off reflecting them.
While longer ones do not have enough energy to be of much use chemically,
so absorbance of them would lead to excessive heating.
But even more importantly, as I said earlier light capture isnít really
where the major "design flaw" of photosynthesis is found. It is in carbon
uptake, and one enzyme in particular is the culprit:
ribulose 1,5 bis-phosphate carboxylase, also known as rubisco. This is the
enzyme that takes a carbon dioxide molecule and adds it to the end of a
short sugar molecule, called
ribulose-1,5-bisphosphate. This enzyme is ancient, and almost certainly
first appeared billions of years ago when photosynthesis was relatively
new, and carbon dioxide made up a large
percentage of the atmosphere, and oxygen a very small part. It is very
slow, for example: while some enzymes may process over a million substrate
molecules a second per active site, bloated
rubisco can only process 2 or 3 per second per active site. And not only is
it slow, it is also horribly non-discriminating in its substrates. It can
react with oxygen instead of carbon dioxide in a
reaction that is not just useless, but also very wasteful. It slows down
carbon dioxide uptake through competitive inhibition, and it causes the
plant to have to go to great lengths and actually
spend energy and give off a carbon dioxide molecule to recycle the sugar
molecule consumed in the process. And while oxygen makes up about 21% of
the gas in our atmosphere, carbon
dioxide makes up much less than a tenth of 1%.
Because of the horrible inefficiency of rubisco, and the low amounts of
carbon dioxide in the atmosphere, the plant has to make lots of rubisco to
be able to fulfill its carbon needs. As a matter
of fact, it is probably the most abundant protein on earth. In a typical
plant, it may actually contain something like 50% of all the nitrogen in
the whole plant. So the plant must balance its needs
for carbon dioxide with its ability to take up nitrogen. And to further
complicate things, the same pores that the plant relies on to "breathe in"
carbon dioxide also let a lot of water evaporate out
of the plant. Some plants actually lose 1500 molecules of water for every
one carbon dioxide that they take up(2). So the plant must also balance its
carbon needs with the water that is available
in its immediate environment. So it is a wonder to me that plants can grow
at all sometimes. Some plants have found ways to somewhat get around the
limitations of rubisco and a low carbon
dioxide atmosphere, but none of them have found a way to totally replace
the enzyme. And neither have genetic engineers, but some of them are
working on improving it in crop plants.
Well, I donít want to make this reply too long. I hope Iíve at least
partially answered your question. If you or your students are interested in
learning more about photosynthesis, here are some
web sites to check out:
Hereís the home page of the lab of Dr. Tony Crofts, where I am working on
my Ph.D. thesis:
http://ahab.life.uiuc.edu/home.h
tml
We work mainly on the bc1 complex, a major component of the electron
transport chain in mitochondria, bacteria, and some kinds of photosynthetic
bacteria. But there are links to pages about
other components of photosynthesis as well.
Hereís a page with structural and mechanistic information about Rubisco,
but I didnít see any information on photorespiration (oxygen uptake by
rubisco, and the pathways used to account for
it):
http://www.rrz.uni-hamburg.de/biologie/ialb/lehre/molbio/1rxo/e1rxoe.h
tm
Hereís a page on carbon fixation:
http://www.epsilon-assoc.com/~jkimball/BiologyPages/C/CalvinCycle.html
Hereís a previous Mad Scientist question on photorespiration:
htt
p://www.madsci.org/posts/archives/may96/828679089.Bt.q.html
Hereís how one group of plants has worked around the photorespiration
problem:
http
://www.marietta.edu/~spilatrs/biol103/photolab/c4photo.html
Also, the light reactions send their electrons to the dark reactions of
photosynthesis. So, in high light conditions the dark may reactions get
backed up due to the inefficiency of rubisco and low
carbon dioxide, and the light reactions can actually find themselves
absorbing too much energy from light and generating an excess of electrons.
They must find some way to get rid of this
excess energy other than photochemistry. This is called non-photochemical
quenching, and hereís a link to a paper on it by Dr. Crofts and Dr.
Christine Yerkes:
http://ahab.life.uiuc.edu/lhcii
.html
1. The figure was copied from notes that Dr. Don Ort gave out in the
Biophysics 332 Photosynthesis class on 2/21/2000.
2. Also from notes given out by Dr. Ort, but on 3/1/2000.
So in this experiment light of particular wavelengths was shined onto a few
materials, and then the amount that got through was measured. The x-axis is
the wavelength of light in nanometers
(10-9 meters), and the y-axis is the amount of light absorbed by the
material being observed. The absorbance is defined as A=log T0/T, where T
is the amount of light transmitted and T0 is the
initial amount of light before passing into the sample. An absorbance of
1.0 means that 90% of the light was absorbed, A=0.5 means about 2/3 was
absorbed, and A=0.0 means that it all got
through. There are two important traces here. One is at the top, and has
data points represented as open boxes. It is from a thin, single leaf. The
other important one is at the bottom and has
open circles. It is the absorbtion spectrum for chlorophyll that has been
extracted from a leaf. As you can see, although chlorophyll by itself
doesnít absorb very well in the green wavelengths
(the low trough on the graph), the whole leaf has A > 0.5, so it absorbs
over 2/3 of the incident light in the green wavelength. Also, we must
consider that most plants are part of a "canopy",
where light first encounters tall plants, and then what isnít absorbed by
the tallest plants goes down to the next level, and so on. So, eventually
almost all of even the green light is absorbed. The
wavelengths scanned here pretty much cover the visible spectrum.
Wavelengths shorter than this carry too much energy and can be actually
harmful, so the plant is better off reflecting them.
While longer ones do not have enough energy to be of much use chemically,
so absorbance of them would lead to excessive heating.
But even more importantly, as I said earlier light capture isnít really
where the major "design flaw" of photosynthesis is found. It is in carbon
uptake, and one enzyme in particular is the culprit:
ribulose 1,5 bis-phosphate carboxylase, also known as rubisco. This is the
enzyme that takes a carbon dioxide molecule and adds it to the end of a
short sugar molecule, called
ribulose-1,5-bisphosphate. This enzyme is ancient, and almost certainly
first appeared billions of years ago when photosynthesis was relatively
new, and carbon dioxide made up a large
percentage of the atmosphere, and oxygen a very small part. It is very
slow, for example: while some enzymes may process over a million substrate
molecules a second per active site, bloated
rubisco can only process 2 or 3 per second per active site. And not only is
it slow, it is also horribly non-discriminating in its substrates. It can
react with oxygen instead of carbon dioxide in a
reaction that is not just useless, but also very wasteful. It slows down
carbon dioxide uptake through competitive inhibition, and it causes the
plant to have to go to great lengths and actually
spend energy and give off a carbon dioxide molecule to recycle the sugar
molecule consumed in the process. And while oxygen makes up about 21% of
the gas in our atmosphere, carbon
dioxide makes up much less than a tenth of 1%.
Because of the horrible inefficiency of rubisco, and the low amounts of
carbon dioxide in the atmosphere, the plant has to make lots of rubisco to
be able to fulfill its carbon needs. As a matter
of fact, it is probably the most abundant protein on earth. In a typical
plant, it may actually contain something like 50% of all the nitrogen in
the whole plant. So the plant must balance its needs
for carbon dioxide with its ability to take up nitrogen. And to further
complicate things, the same pores that the plant relies on to "breathe in"
carbon dioxide also let a lot of water evaporate out
of the plant. Some plants actually lose 1500 molecules of water for every
one carbon dioxide that they take up(2). So the plant must also balance its
carbon needs with the water that is available
in its immediate environment. So it is a wonder to me that plants can grow
at all sometimes. Some plants have found ways to somewhat get around the
limitations of rubisco and a low carbon
dioxide atmosphere, but none of them have found a way to totally replace
the enzyme. And neither have genetic engineers, but some of them are
working on improving it in crop plants.
Well, I donít want to make this reply too long. I hope Iíve at least
partially answered your question. If you or your students are interested in
learning more about photosynthesis, here are some
web sites to check out:
Hereís the home page of the lab of Dr. Tony Crofts, where I am working on
my Ph.D. thesis:
http://ahab.life.uiuc.edu/home.h
tml
We work mainly on the bc1 complex, a major component of the electron
transport chain in mitochondria, bacteria, and some kinds of photosynthetic
bacteria. But there are links to pages about
other components of photosynthesis as well.
Hereís a page with structural and mechanistic information about Rubisco,
but I didnít see any information on photorespiration (oxygen uptake by
rubisco, and the pathways used to account for
it):
http://www.rrz.uni-hamburg.de/biologie/ialb/lehre/molbio/1rxo/e1rxoe.h
tm
Hereís a page on carbon fixation:
http://www.epsilon-assoc.com/~jkimball/BiologyPages/C/CalvinCycle.html
Hereís a previous Mad Scientist question on photorespiration:
htt
p://www.madsci.org/posts/archives/may96/828679089.Bt.q.html
Hereís how one group of plants has worked around the photorespiration
problem:
http
://www.marietta.edu/~spilatrs/biol103/photolab/c4photo.html
Also, the light reactions send their electrons to the dark reactions of
photosynthesis. So, in high light conditions the dark may reactions get
backed up due to the inefficiency of rubisco and low
carbon dioxide, and the light reactions can actually find themselves
absorbing too much energy from light and generating an excess of electrons.
They must find some way to get rid of this
excess energy other than photochemistry. This is called non-photochemical
quenching, and hereís a link to a paper on it by Dr. Crofts and Dr.
Christine Yerkes:
http://ahab.life.uiuc.edu/lhcii
.html
1. The figure was copied from notes that Dr. Don Ort gave out in the
Biophysics 332 Photosynthesis class on 2/21/2000.
2. Also from notes given out by Dr. Ort, but on 3/1/2000.
Current Queue |
Current Queue for Botany |
Botany archives
Try the links in the MadSci Library for more information on Botany.
MadSci Home | Information |
Search |
Random Knowledge Generator |
MadSci Archives |
Mad Library | MAD Labs |
MAD FAQs |
Ask a ? |
Join Us! |
Help Support MadSci
MadSci Network,
webadmin@www.madsci.org
© 1995-2000. All rights reserved.
 So in this experiment light of particular wavelengths was shined onto a few
materials, and then the amount that got through was measured. The x-axis is
the wavelength of light in nanometers
(10-9 meters), and the y-axis is the amount of light absorbed by the
material being observed. The absorbance is defined as A=log T0/T, where T
is the amount of light transmitted and T0 is the
initial amount of light before passing into the sample. An absorbance of
1.0 means that 90% of the light was absorbed, A=0.5 means about 2/3 was
absorbed, and A=0.0 means that it all got
through. There are two important traces here. One is at the top, and has
data points represented as open boxes. It is from a thin, single leaf. The
other important one is at the bottom and has
open circles. It is the absorbtion spectrum for chlorophyll that has been
extracted from a leaf. As you can see, although chlorophyll by itself
doesnít absorb very well in the green wavelengths
(the low trough on the graph), the whole leaf has A > 0.5, so it absorbs
over 2/3 of the incident light in the green wavelength. Also, we must
consider that most plants are part of a "canopy",
where light first encounters tall plants, and then what isnít absorbed by
the tallest plants goes down to the next level, and so on. So, eventually
almost all of even the green light is absorbed. The
wavelengths scanned here pretty much cover the visible spectrum.
Wavelengths shorter than this carry too much energy and can be actually
harmful, so the plant is better off reflecting them.
While longer ones do not have enough energy to be of much use chemically,
so absorbance of them would lead to excessive heating.
But even more importantly, as I said earlier light capture isnít really
where the major "design flaw" of photosynthesis is found. It is in carbon
uptake, and one enzyme in particular is the culprit:
ribulose 1,5 bis-phosphate carboxylase, also known as rubisco. This is the
enzyme that takes a carbon dioxide molecule and adds it to the end of a
short sugar molecule, called
ribulose-1,5-bisphosphate. This enzyme is ancient, and almost certainly
first appeared billions of years ago when photosynthesis was relatively
new, and carbon dioxide made up a large
percentage of the atmosphere, and oxygen a very small part. It is very
slow, for example: while some enzymes may process over a million substrate
molecules a second per active site, bloated
rubisco can only process 2 or 3 per second per active site. And not only is
it slow, it is also horribly non-discriminating in its substrates. It can
react with oxygen instead of carbon dioxide in a
reaction that is not just useless, but also very wasteful. It slows down
carbon dioxide uptake through competitive inhibition, and it causes the
plant to have to go to great lengths and actually
spend energy and give off a carbon dioxide molecule to recycle the sugar
molecule consumed in the process. And while oxygen makes up about 21% of
the gas in our atmosphere, carbon
dioxide makes up much less than a tenth of 1%.
Because of the horrible inefficiency of rubisco, and the low amounts of
carbon dioxide in the atmosphere, the plant has to make lots of rubisco to
be able to fulfill its carbon needs. As a matter
of fact, it is probably the most abundant protein on earth. In a typical
plant, it may actually contain something like 50% of all the nitrogen in
the whole plant. So the plant must balance its needs
for carbon dioxide with its ability to take up nitrogen. And to further
complicate things, the same pores that the plant relies on to "breathe in"
carbon dioxide also let a lot of water evaporate out
of the plant. Some plants actually lose 1500 molecules of water for every
one carbon dioxide that they take up(2). So the plant must also balance its
carbon needs with the water that is available
in its immediate environment. So it is a wonder to me that plants can grow
at all sometimes. Some plants have found ways to somewhat get around the
limitations of rubisco and a low carbon
dioxide atmosphere, but none of them have found a way to totally replace
the enzyme. And neither have genetic engineers, but some of them are
working on improving it in crop plants.
Well, I donít want to make this reply too long. I hope Iíve at least
partially answered your question. If you or your students are interested in
learning more about photosynthesis, here are some
web sites to check out:
Hereís the home page of the lab of Dr. Tony Crofts, where I am working on
my Ph.D. thesis:
http://ahab.life.uiuc.edu/home.h
tml
We work mainly on the bc1 complex, a major component of the electron
transport chain in mitochondria, bacteria, and some kinds of photosynthetic
bacteria. But there are links to pages about
other components of photosynthesis as well.
Hereís a page with structural and mechanistic information about Rubisco,
but I didnít see any information on photorespiration (oxygen uptake by
rubisco, and the pathways used to account for
it):
http://www.rrz.uni-hamburg.de/biologie/ialb/lehre/molbio/1rxo/e1rxoe.h
tm
Hereís a page on carbon fixation:
http://www.epsilon-assoc.com/~jkimball/BiologyPages/C/CalvinCycle.html
Hereís a previous Mad Scientist question on photorespiration:
htt
p://www.madsci.org/posts/archives/may96/828679089.Bt.q.html
Hereís how one group of plants has worked around the photorespiration
problem:
http
://www.marietta.edu/~spilatrs/biol103/photolab/c4photo.html
Also, the light reactions send their electrons to the dark reactions of
photosynthesis. So, in high light conditions the dark may reactions get
backed up due to the inefficiency of rubisco and low
carbon dioxide, and the light reactions can actually find themselves
absorbing too much energy from light and generating an excess of electrons.
They must find some way to get rid of this
excess energy other than photochemistry. This is called non-photochemical
quenching, and hereís a link to a paper on it by Dr. Crofts and Dr.
Christine Yerkes:
http://ahab.life.uiuc.edu/lhcii
.html
1. The figure was copied from notes that Dr. Don Ort gave out in the
Biophysics 332 Photosynthesis class on 2/21/2000.
2. Also from notes given out by Dr. Ort, but on 3/1/2000.
So in this experiment light of particular wavelengths was shined onto a few
materials, and then the amount that got through was measured. The x-axis is
the wavelength of light in nanometers
(10-9 meters), and the y-axis is the amount of light absorbed by the
material being observed. The absorbance is defined as A=log T0/T, where T
is the amount of light transmitted and T0 is the
initial amount of light before passing into the sample. An absorbance of
1.0 means that 90% of the light was absorbed, A=0.5 means about 2/3 was
absorbed, and A=0.0 means that it all got
through. There are two important traces here. One is at the top, and has
data points represented as open boxes. It is from a thin, single leaf. The
other important one is at the bottom and has
open circles. It is the absorbtion spectrum for chlorophyll that has been
extracted from a leaf. As you can see, although chlorophyll by itself
doesnít absorb very well in the green wavelengths
(the low trough on the graph), the whole leaf has A > 0.5, so it absorbs
over 2/3 of the incident light in the green wavelength. Also, we must
consider that most plants are part of a "canopy",
where light first encounters tall plants, and then what isnít absorbed by
the tallest plants goes down to the next level, and so on. So, eventually
almost all of even the green light is absorbed. The
wavelengths scanned here pretty much cover the visible spectrum.
Wavelengths shorter than this carry too much energy and can be actually
harmful, so the plant is better off reflecting them.
While longer ones do not have enough energy to be of much use chemically,
so absorbance of them would lead to excessive heating.
But even more importantly, as I said earlier light capture isnít really
where the major "design flaw" of photosynthesis is found. It is in carbon
uptake, and one enzyme in particular is the culprit:
ribulose 1,5 bis-phosphate carboxylase, also known as rubisco. This is the
enzyme that takes a carbon dioxide molecule and adds it to the end of a
short sugar molecule, called
ribulose-1,5-bisphosphate. This enzyme is ancient, and almost certainly
first appeared billions of years ago when photosynthesis was relatively
new, and carbon dioxide made up a large
percentage of the atmosphere, and oxygen a very small part. It is very
slow, for example: while some enzymes may process over a million substrate
molecules a second per active site, bloated
rubisco can only process 2 or 3 per second per active site. And not only is
it slow, it is also horribly non-discriminating in its substrates. It can
react with oxygen instead of carbon dioxide in a
reaction that is not just useless, but also very wasteful. It slows down
carbon dioxide uptake through competitive inhibition, and it causes the
plant to have to go to great lengths and actually
spend energy and give off a carbon dioxide molecule to recycle the sugar
molecule consumed in the process. And while oxygen makes up about 21% of
the gas in our atmosphere, carbon
dioxide makes up much less than a tenth of 1%.
Because of the horrible inefficiency of rubisco, and the low amounts of
carbon dioxide in the atmosphere, the plant has to make lots of rubisco to
be able to fulfill its carbon needs. As a matter
of fact, it is probably the most abundant protein on earth. In a typical
plant, it may actually contain something like 50% of all the nitrogen in
the whole plant. So the plant must balance its needs
for carbon dioxide with its ability to take up nitrogen. And to further
complicate things, the same pores that the plant relies on to "breathe in"
carbon dioxide also let a lot of water evaporate out
of the plant. Some plants actually lose 1500 molecules of water for every
one carbon dioxide that they take up(2). So the plant must also balance its
carbon needs with the water that is available
in its immediate environment. So it is a wonder to me that plants can grow
at all sometimes. Some plants have found ways to somewhat get around the
limitations of rubisco and a low carbon
dioxide atmosphere, but none of them have found a way to totally replace
the enzyme. And neither have genetic engineers, but some of them are
working on improving it in crop plants.
Well, I donít want to make this reply too long. I hope Iíve at least
partially answered your question. If you or your students are interested in
learning more about photosynthesis, here are some
web sites to check out:
Hereís the home page of the lab of Dr. Tony Crofts, where I am working on
my Ph.D. thesis:
http://ahab.life.uiuc.edu/home.h
tml
We work mainly on the bc1 complex, a major component of the electron
transport chain in mitochondria, bacteria, and some kinds of photosynthetic
bacteria. But there are links to pages about
other components of photosynthesis as well.
Hereís a page with structural and mechanistic information about Rubisco,
but I didnít see any information on photorespiration (oxygen uptake by
rubisco, and the pathways used to account for
it):
http://www.rrz.uni-hamburg.de/biologie/ialb/lehre/molbio/1rxo/e1rxoe.h
tm
Hereís a page on carbon fixation:
http://www.epsilon-assoc.com/~jkimball/BiologyPages/C/CalvinCycle.html
Hereís a previous Mad Scientist question on photorespiration:
htt
p://www.madsci.org/posts/archives/may96/828679089.Bt.q.html
Hereís how one group of plants has worked around the photorespiration
problem:
http
://www.marietta.edu/~spilatrs/biol103/photolab/c4photo.html
Also, the light reactions send their electrons to the dark reactions of
photosynthesis. So, in high light conditions the dark may reactions get
backed up due to the inefficiency of rubisco and low
carbon dioxide, and the light reactions can actually find themselves
absorbing too much energy from light and generating an excess of electrons.
They must find some way to get rid of this
excess energy other than photochemistry. This is called non-photochemical
quenching, and hereís a link to a paper on it by Dr. Crofts and Dr.
Christine Yerkes:
http://ahab.life.uiuc.edu/lhcii
.html
1. The figure was copied from notes that Dr. Don Ort gave out in the
Biophysics 332 Photosynthesis class on 2/21/2000.
2. Also from notes given out by Dr. Ort, but on 3/1/2000.