e.g: chlorine atom has a radius of 0.099 nm . Its anion has a radius of 0.181 nm (almost double).
Three words: electron-electron repulsion.
The more electrons you pack into the valence shell, the more electron-electron repulsion there is within that shell--after all, the electrons are all negatively charged! In neutral atoms this is balanced by an increase in the (positive) nuclear charge, and atomic size actually decreases as you move from left to right in any row of the periodic table.
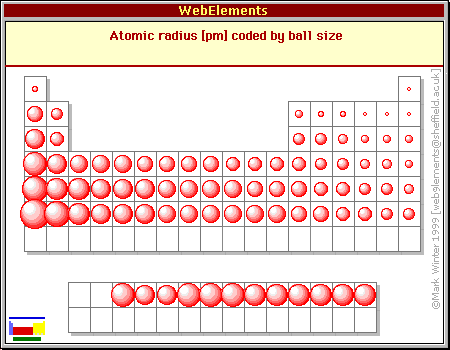
Chart courtesy of WebElements. For more on why atomic sizes decrease as you move left-to-right in a period, see this MadSci answer.
But when going from a chlorine atom to a chlorine anion, there is no increase in nuclear charge. With nothing to counterbalance the increased electron-electron repulsion, the ion balloons in size relative to the neutral atom.
| Dan Berger |
| Bluffton College |
| http://cs.bluffton.edu/~berger |