 Ethylene, CH2=CH2
Ethylene, CH2=CH2
| MadSci Network: Chemistry |
This is an excellent question, but one that is rather hard to answer at the high school level. Here goes...
Double bonds have electron density above and below the plane of the double- bond system, a "pi-system" of electrons:
 Ethylene, CH2=CH2
Ethylene, CH2=CH2
A system of alternating C-C single and C-C double bonds ("conjugated" double bonds) can overlap and extend the pi-system:
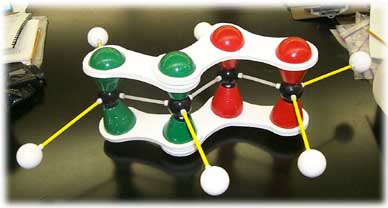 Butadiene, CH2=CH-CH=CH2
Butadiene, CH2=CH-CH=CH2
This is stronger than 2 isolated double bonds.
When two double bonds are next to each other ("cumulated" double bonds), the pi-electrons cannot overlap, but are perpendicular to each other:
 Allene, CH2=C=CH2
Allene, CH2=C=CH2
This is considerably less stable than 2 isolated double bonds.
Given any opportunity, such as uv light or free radicals in the environment, cumulated double bonds will rearrange to conjugated double bonds.
A long chain of cumulated double bonds:
... C=C=C=C=C=C=C=C ...
would be particularly reactive.
In many respects, this is equivalent to conjugated triple bonds, which are also unstable.
 Acetylene, HCCH, a molecule that contains a C-C triple bond
Acetylene, HCCH, a molecule that contains a C-C triple bond
Source for most of this material: Morrison and Boyd, "Organic Chemistry", 4th edition.
Try the links in the MadSci Library for more information on Chemistry.