Date: Mon Aug 14 02:05:25 2000
Posted By: Bradley Kelley, Grad student, Mechanical Engineering, Colorado State University
Area of science: General Biology
ID: 963886526.Gb
Message:
Glenn,
You are correct in saying that too much oxygen can be poisonous. Breathing
100% oxygen at sea level pressure can cause oxygen toxicity in
approximately 1 hour. One of the first noticeable symptoms of oxygen
toxicity is seizure, so it can be very dangerous, especially for divers.
Much if my information I attained from the following web-sight which is a
book by Dr. Lawrence Martin, M.D. on diving. http://www
.mtsinai.org/pulmonary/books/scuba/contents.htm
I was unable to confirm the use of 100% oxygen on the Apollo flights but I
used the information from the dive sight to figure out how they would do
it. By the way, I am guessing that the reason they used pure oxygen was
for weight savings. Very important in space flight (well, at least the
launch part!) Air is approximately 21% oxygen, so using air instead of
oxygen would have weighed nearly 5 times that of the pure oxygen.
To get back to your question, the important rule is Henry's Law as quoted
from Dr. Martin's book. (Section D "An Explanation of Pressure and the Laws
of Dalton, Boyle, Charles and Henry")
"Henry's law states:
The amount of any gas that will dissolve in a liquid at a given temperature
is a function of the partial pressure of the gas in contact with the liquid
and the solubility coefficient of the gas in that particular liquid.
Simplified: As the pressure of any gas increases, more of that gas will
dissolve into any solution with which it is in free contact.
Mathematically,
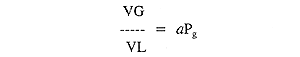 where VG is the volume of a particular gas, VL is the volume of a
particular liquid, a is the solubility coefficient for the gas in that
liquid, and Pg is the pressure of the gas in contact with the liquid.
Taken together, Henry and Dalton's laws predict two very important
consequences:
1) When ambient pressure is lowered (as at altitude), the partial pressure
of oxygen and nitrogen in the body must fall, and there will be less
molecules of each gas dissolved in the blood and tissues.
2) When ambient pressure is raised (as when diving), the partial pressure
of oxygen and nitrogen in the body must rise, and there will be more
molecules of each gas dissolved in the blood and tissues."
Your blood carries oxygen in two ways, attached to the hemoglobin of the
red blood cells and dissolved in the plasma. Each red blood cell carries
at max 4 oxygen molecules so increasing or decreasing the pressure won't
affect that much, but changing the pressure will affect the amount of gas
in the plasma. This has serious consequences for divers but is also how
the astronauts were able to breathe 100% oxygen without toxic effects.
At sea level we live at approximately 14.7 psi of pressure from the weight
of the air above us (Also known as 1 atmosphere). At this pressure our
plasma has a certain amount of oxygen and nitrogen (as well as CO2 and
other gasses) dissolved in it. Henry's law shows that if we increase the
ambient pressure (the pressure that your body and lungs feel) then the
plasma is able to hold more molecules of each gas. This can be serious for
divers. If someone dives to 33 feet under water they are at approximately
2 atmospheres, or twice the pressure. Now I don't think the plasma can
directly hold exactly twice as many molecules, but it certainly can hold
more. Why is this dangerous? If a diver has been under greater than
atmospheric pressure for an extended time to allow more gas to dissolve
into the blood than suddenly goes from high pressure to a lower pressure,
the plasma can no longer hold as much gas molecules and they will form back
into a gas (while still in you). This condition is known as 'the bends' to
divers. A great example of this is to open a soda can. Soda is under a
positive pressure, which allows the pop to hold more molecules of CO2 than
when the top is released and the liquid is now at atmospheric pressure and
CO2 is released.
So now we have an example of increasing pressure affecting the gas in the
blood, but Henry's law also shows that decreasing the pressure will
decrease the amount of oxygen in the blood. Here in Colorado we are at 1
mile above sea level (about .75 Atmospheres) and I will have less oxygen
dissolved in my blood then you will there in Connecticut. (I believe my
body compensates by increasing the number of red blood cells, but don't
quote me on that because I didn't look it up). But this is because we
breathe the same air with the same percentage of oxygen. If I were to
increase the percentage of oxygen, I would soon be able to match the amount
of dissolved oxygen that you at sea level would have. At this lesser
pressure I will have less total molecules of dissolved air, but a greater
percentage of these dissolve molecules will be oxygen. This is how the
Apollo space program was able to give the astronauts 100% oxygen for an
extended period of time. At the low pressure the plasma in the blood will
hold a lot less total molecules than you and I, but nearly all of them will
be oxygen, unlike us air breathers with nearly 78% of the total dissolved
molecules being nitrogen. Hope this made sense. The given website is an
excellent source if you have more questions about blood-gas and other
respiratory questions. Good luck.
BK
where VG is the volume of a particular gas, VL is the volume of a
particular liquid, a is the solubility coefficient for the gas in that
liquid, and Pg is the pressure of the gas in contact with the liquid.
Taken together, Henry and Dalton's laws predict two very important
consequences:
1) When ambient pressure is lowered (as at altitude), the partial pressure
of oxygen and nitrogen in the body must fall, and there will be less
molecules of each gas dissolved in the blood and tissues.
2) When ambient pressure is raised (as when diving), the partial pressure
of oxygen and nitrogen in the body must rise, and there will be more
molecules of each gas dissolved in the blood and tissues."
Your blood carries oxygen in two ways, attached to the hemoglobin of the
red blood cells and dissolved in the plasma. Each red blood cell carries
at max 4 oxygen molecules so increasing or decreasing the pressure won't
affect that much, but changing the pressure will affect the amount of gas
in the plasma. This has serious consequences for divers but is also how
the astronauts were able to breathe 100% oxygen without toxic effects.
At sea level we live at approximately 14.7 psi of pressure from the weight
of the air above us (Also known as 1 atmosphere). At this pressure our
plasma has a certain amount of oxygen and nitrogen (as well as CO2 and
other gasses) dissolved in it. Henry's law shows that if we increase the
ambient pressure (the pressure that your body and lungs feel) then the
plasma is able to hold more molecules of each gas. This can be serious for
divers. If someone dives to 33 feet under water they are at approximately
2 atmospheres, or twice the pressure. Now I don't think the plasma can
directly hold exactly twice as many molecules, but it certainly can hold
more. Why is this dangerous? If a diver has been under greater than
atmospheric pressure for an extended time to allow more gas to dissolve
into the blood than suddenly goes from high pressure to a lower pressure,
the plasma can no longer hold as much gas molecules and they will form back
into a gas (while still in you). This condition is known as 'the bends' to
divers. A great example of this is to open a soda can. Soda is under a
positive pressure, which allows the pop to hold more molecules of CO2 than
when the top is released and the liquid is now at atmospheric pressure and
CO2 is released.
So now we have an example of increasing pressure affecting the gas in the
blood, but Henry's law also shows that decreasing the pressure will
decrease the amount of oxygen in the blood. Here in Colorado we are at 1
mile above sea level (about .75 Atmospheres) and I will have less oxygen
dissolved in my blood then you will there in Connecticut. (I believe my
body compensates by increasing the number of red blood cells, but don't
quote me on that because I didn't look it up). But this is because we
breathe the same air with the same percentage of oxygen. If I were to
increase the percentage of oxygen, I would soon be able to match the amount
of dissolved oxygen that you at sea level would have. At this lesser
pressure I will have less total molecules of dissolved air, but a greater
percentage of these dissolve molecules will be oxygen. This is how the
Apollo space program was able to give the astronauts 100% oxygen for an
extended period of time. At the low pressure the plasma in the blood will
hold a lot less total molecules than you and I, but nearly all of them will
be oxygen, unlike us air breathers with nearly 78% of the total dissolved
molecules being nitrogen. Hope this made sense. The given website is an
excellent source if you have more questions about blood-gas and other
respiratory questions. Good luck.
BK
Current Queue |
Current Queue for General Biology |
General Biology archives
Try the links in the MadSci Library for more information on General Biology.
MadSci Home | Information |
Search |
Random Knowledge Generator |
MadSci Archives |
Mad Library | MAD Labs |
MAD FAQs |
Ask a ? |
Join Us! |
Help Support MadSci
MadSci Network,
webadmin@www.madsci.org
© 1995-2000. All rights reserved.
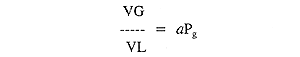 where VG is the volume of a particular gas, VL is the volume of a
particular liquid, a is the solubility coefficient for the gas in that
liquid, and Pg is the pressure of the gas in contact with the liquid.
Taken together, Henry and Dalton's laws predict two very important
consequences:
1) When ambient pressure is lowered (as at altitude), the partial pressure
of oxygen and nitrogen in the body must fall, and there will be less
molecules of each gas dissolved in the blood and tissues.
2) When ambient pressure is raised (as when diving), the partial pressure
of oxygen and nitrogen in the body must rise, and there will be more
molecules of each gas dissolved in the blood and tissues."
Your blood carries oxygen in two ways, attached to the hemoglobin of the
red blood cells and dissolved in the plasma. Each red blood cell carries
at max 4 oxygen molecules so increasing or decreasing the pressure won't
affect that much, but changing the pressure will affect the amount of gas
in the plasma. This has serious consequences for divers but is also how
the astronauts were able to breathe 100% oxygen without toxic effects.
At sea level we live at approximately 14.7 psi of pressure from the weight
of the air above us (Also known as 1 atmosphere). At this pressure our
plasma has a certain amount of oxygen and nitrogen (as well as CO2 and
other gasses) dissolved in it. Henry's law shows that if we increase the
ambient pressure (the pressure that your body and lungs feel) then the
plasma is able to hold more molecules of each gas. This can be serious for
divers. If someone dives to 33 feet under water they are at approximately
2 atmospheres, or twice the pressure. Now I don't think the plasma can
directly hold exactly twice as many molecules, but it certainly can hold
more. Why is this dangerous? If a diver has been under greater than
atmospheric pressure for an extended time to allow more gas to dissolve
into the blood than suddenly goes from high pressure to a lower pressure,
the plasma can no longer hold as much gas molecules and they will form back
into a gas (while still in you). This condition is known as 'the bends' to
divers. A great example of this is to open a soda can. Soda is under a
positive pressure, which allows the pop to hold more molecules of CO2 than
when the top is released and the liquid is now at atmospheric pressure and
CO2 is released.
So now we have an example of increasing pressure affecting the gas in the
blood, but Henry's law also shows that decreasing the pressure will
decrease the amount of oxygen in the blood. Here in Colorado we are at 1
mile above sea level (about .75 Atmospheres) and I will have less oxygen
dissolved in my blood then you will there in Connecticut. (I believe my
body compensates by increasing the number of red blood cells, but don't
quote me on that because I didn't look it up). But this is because we
breathe the same air with the same percentage of oxygen. If I were to
increase the percentage of oxygen, I would soon be able to match the amount
of dissolved oxygen that you at sea level would have. At this lesser
pressure I will have less total molecules of dissolved air, but a greater
percentage of these dissolve molecules will be oxygen. This is how the
Apollo space program was able to give the astronauts 100% oxygen for an
extended period of time. At the low pressure the plasma in the blood will
hold a lot less total molecules than you and I, but nearly all of them will
be oxygen, unlike us air breathers with nearly 78% of the total dissolved
molecules being nitrogen. Hope this made sense. The given website is an
excellent source if you have more questions about blood-gas and other
respiratory questions. Good luck.
BK
where VG is the volume of a particular gas, VL is the volume of a
particular liquid, a is the solubility coefficient for the gas in that
liquid, and Pg is the pressure of the gas in contact with the liquid.
Taken together, Henry and Dalton's laws predict two very important
consequences:
1) When ambient pressure is lowered (as at altitude), the partial pressure
of oxygen and nitrogen in the body must fall, and there will be less
molecules of each gas dissolved in the blood and tissues.
2) When ambient pressure is raised (as when diving), the partial pressure
of oxygen and nitrogen in the body must rise, and there will be more
molecules of each gas dissolved in the blood and tissues."
Your blood carries oxygen in two ways, attached to the hemoglobin of the
red blood cells and dissolved in the plasma. Each red blood cell carries
at max 4 oxygen molecules so increasing or decreasing the pressure won't
affect that much, but changing the pressure will affect the amount of gas
in the plasma. This has serious consequences for divers but is also how
the astronauts were able to breathe 100% oxygen without toxic effects.
At sea level we live at approximately 14.7 psi of pressure from the weight
of the air above us (Also known as 1 atmosphere). At this pressure our
plasma has a certain amount of oxygen and nitrogen (as well as CO2 and
other gasses) dissolved in it. Henry's law shows that if we increase the
ambient pressure (the pressure that your body and lungs feel) then the
plasma is able to hold more molecules of each gas. This can be serious for
divers. If someone dives to 33 feet under water they are at approximately
2 atmospheres, or twice the pressure. Now I don't think the plasma can
directly hold exactly twice as many molecules, but it certainly can hold
more. Why is this dangerous? If a diver has been under greater than
atmospheric pressure for an extended time to allow more gas to dissolve
into the blood than suddenly goes from high pressure to a lower pressure,
the plasma can no longer hold as much gas molecules and they will form back
into a gas (while still in you). This condition is known as 'the bends' to
divers. A great example of this is to open a soda can. Soda is under a
positive pressure, which allows the pop to hold more molecules of CO2 than
when the top is released and the liquid is now at atmospheric pressure and
CO2 is released.
So now we have an example of increasing pressure affecting the gas in the
blood, but Henry's law also shows that decreasing the pressure will
decrease the amount of oxygen in the blood. Here in Colorado we are at 1
mile above sea level (about .75 Atmospheres) and I will have less oxygen
dissolved in my blood then you will there in Connecticut. (I believe my
body compensates by increasing the number of red blood cells, but don't
quote me on that because I didn't look it up). But this is because we
breathe the same air with the same percentage of oxygen. If I were to
increase the percentage of oxygen, I would soon be able to match the amount
of dissolved oxygen that you at sea level would have. At this lesser
pressure I will have less total molecules of dissolved air, but a greater
percentage of these dissolve molecules will be oxygen. This is how the
Apollo space program was able to give the astronauts 100% oxygen for an
extended period of time. At the low pressure the plasma in the blood will
hold a lot less total molecules than you and I, but nearly all of them will
be oxygen, unlike us air breathers with nearly 78% of the total dissolved
molecules being nitrogen. Hope this made sense. The given website is an
excellent source if you have more questions about blood-gas and other
respiratory questions. Good luck.
BK