Date: Sat Mar 24 18:11:21 2001
Posted By: David Reibstein, Associate Dean
Area of science: Engineering
ID: 985048933.Eg
Message:
Question: how does a curling iron curl hair?
 Hair is made almost entirely of protein. The particular kind
of protein
that makes up hair is called a-keratin
(alpha-keratin). This protein takes on a shape known as a
helix, in
which the protein strand twists upon itself.
Hair is made almost entirely of protein. The particular kind
of protein
that makes up hair is called a-keratin
(alpha-keratin). This protein takes on a shape known as a
helix, in
which the protein strand twists upon itself.
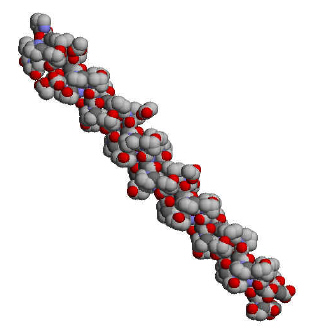 Many proteins adopt this shape, but the particular property of
a-keratin
is that the twisting doesn't stop there. Two helices coil around each
other to form what we call a "coiled coil." And it doesn't
stop
there. Two or more of these coiled coils wrap around each other to
form a filament,
and these filaments wrap around each other to form a structure very much
like a rope,
something like this:
Many proteins adopt this shape, but the particular property of
a-keratin
is that the twisting doesn't stop there. Two helices coil around each
other to form what we call a "coiled coil." And it doesn't
stop
there. Two or more of these coiled coils wrap around each other to
form a filament,
and these filaments wrap around each other to form a structure very much
like a rope,
something like this:
What is holding all of this together? Answer: two types of
chemical
bond. The weaker kind are called hydrogen bonds. The
stronger
are known as disulfide bonds, and these are the kind involved when a
curling iron is used. Disulfide bonds are bonds between two sulfur (S)
atoms on two adjacent strands of the "rope." When hair
curls
into the shape characteristic for a particular person, this shape is held
in
place by the disulfide bonds. When you use a curling iron, you break
the
disulfide bonds. You then wrap the hair around curlers and form into
into
a shape you desire. New disulfide bonds form to hold this new shape
together. When the heat is removed, this shape remains.
As for the exact temperature at which hair starts to curl and so forth,
I'm
afraid I can't help you with that - you need to see a hairdresser.
You can find more information about this subject in standard
biochemistry
textbooks, including:
David Reibstein, Associate Dean,
Albert Dorman Honors College, New Jersey Institute of Technology
Current Queue |
Current Queue for Engineering |
Engineering archives
Try the links in the MadSci Library for more information on Engineering.
MadSci Home | Information |
Search |
Random Knowledge Generator |
MadSci Archives |
Mad Library | MAD Labs |
MAD FAQs |
Ask a ? |
Join Us! |
Help Support MadSci
MadSci Network,
webadmin@www.madsci.org
© 1995-2001. All rights reserved.
 Many proteins adopt this shape, but the particular property of
a-keratin
is that the twisting doesn't stop there. Two helices coil around each
other to form what we call a "coiled coil." And it doesn't
stop
there. Two or more of these coiled coils wrap around each other to
form a filament,
and these filaments wrap around each other to form a structure very much
like a rope,
something like this:
Many proteins adopt this shape, but the particular property of
a-keratin
is that the twisting doesn't stop there. Two helices coil around each
other to form what we call a "coiled coil." And it doesn't
stop
there. Two or more of these coiled coils wrap around each other to
form a filament,
and these filaments wrap around each other to form a structure very much
like a rope,
something like this: Hair is made almost entirely of protein. The particular kind
of protein
that makes up hair is called a-keratin
(alpha-keratin). This protein takes on a shape known as a
helix, in
which the protein strand twists upon itself.
Hair is made almost entirely of protein. The particular kind
of protein
that makes up hair is called a-keratin
(alpha-keratin). This protein takes on a shape known as a
helix, in
which the protein strand twists upon itself.