The tetrathionate ion is S4O62-.
The way to get oxidation numbers for an element combined with oxygen is to assign each oxygen atom an oxidation state of -2. Since the total oxidation state for tetrathionate is -2, and there are six oxygens, each with an oxidation state of -2 for a total of -12, the sum of the sulfur oxidation states has to be equal to -2 - (-12) = +10. Since there are four sulfur atoms, their AVERAGE oxidation state is 10 ¸ 4 = +2.5.
A fractional oxidation state is a dead giveaway that not all the sulfur atoms have the same oxidation state. For example, if two of the sulfur atoms were +3 and the other two were +2, the total would be +10 and the average would be +2.5. For the actual oxidation states, let's look at the Lewis structure of tetrathionate dianion, shown below right.
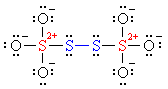 The two sulfurs shown in red are each bonded
to three oxygens and one sulfur, for an oxidation of +3; but they also
carry a +2 formal charge, so their oxidation state is +5. On the other
hand, the two sulfurs shown in blue are bonded
only to sulfur, and have no formal charge; their oxidation state is 0
(zero). The total oxidation states of the four sulfur atoms is
5 + 5 + 0 + 0 = 10, or an average
of +2.5.
The two sulfurs shown in red are each bonded
to three oxygens and one sulfur, for an oxidation of +3; but they also
carry a +2 formal charge, so their oxidation state is +5. On the other
hand, the two sulfurs shown in blue are bonded
only to sulfur, and have no formal charge; their oxidation state is 0
(zero). The total oxidation states of the four sulfur atoms is
5 + 5 + 0 + 0 = 10, or an average
of +2.5.
Remember that "oxidation state" is a formalism! For some purposes, the average oxidation state of sulfur would be more valuable; for others, you would want the individual oxidation states.
| Dan Berger |
| Bluffton College |
| http://www.bluffton.edu/~bergerd |