|
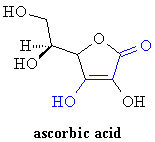
I have a student who is comparing ascorbic acid and citric acid as pH modifiers in an experiment to look at denaturing of the bromelain enzyme in pineapple. On consideration of the structure of ascorbic acid to explain its effect, I see it has no carboxyl group, only hydroxyl groups and a carbonyl group. Does the carbonyl group on an adjacent carbon sufficiently withdraw and delocalise electrons to cause the dissociation of the hydroxyl group on the next carbon atom to cause the acidity of ascorbic acid?
You're on the right track.
Acids are stronger if their conjugate bases are more stable. For example, HF is a stronger acid than H2O because F- is a more stable anion than HO-.
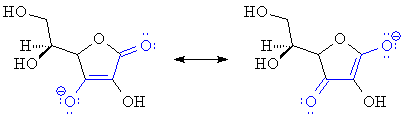
One thing that stabilizes anions is delocalization of the negative charge by resonance (compare the acidities of phenol and cyclohexanol, for example). In ascorbate ion (the conjugate base of ascorbic acid), the negative charge is shared between the two oxygens marked in blue on the structure, by way of the intervening carbon atoms:
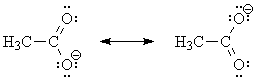
| Bluffton College |
| http://www.bluffton.edu/~bergerd |