I know that B in BF3 does not satisfy the octet rule, so it should form a multicenter bond like BH3 does.... but it doesn't. I think it has something to do with the electronegativity of F but I am not sure. Thanks for any help you can give, I am really stumped.
Actually, the B in BF3 DOES satisfy the octet rule, by electron donation from the three fluorines:
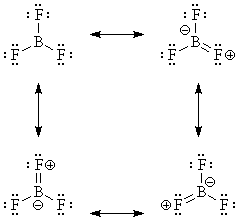
That's why BF3 is monomeric.
In BH3, the only available electron donation is from a B-H bond in a neighboring BH3 molecule:
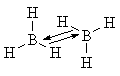 |
which is usually shown as | 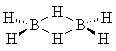 |
| Dan Berger |
| Bluffton College |
| http://www.bluffton.edu/~bergerd |