| MadSci Network: Biochemistry |
Hi Bethan,
Thanks for asking such a great question. I've wondered about this myself too, and I learned quite alot in researching this answer for you.
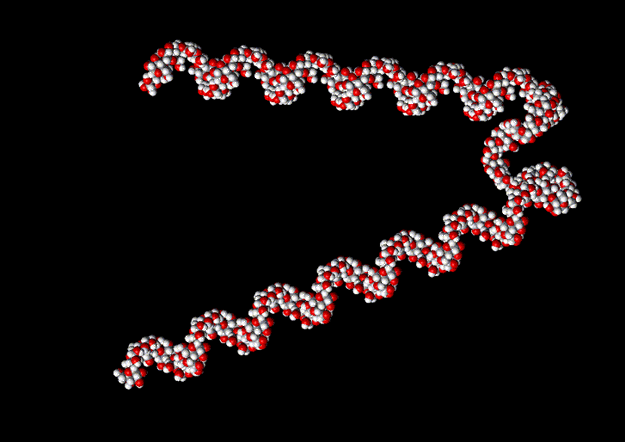
Plant starches are composed of two kinds of polysaccharides, amylose and amylopectin. Both molecules are polymers of sugar (glucose) monomers, used by plants as energy reserves, and the difference between them is that amylose polymers tend to be linear while amylopectin polymers tend to be branched. You can find alot of information about the details of the linkages between the sugar monomers that make up these polymers by doing an internet search for "amylose structure" and "amylopectin structure" or by looking through any biochemistry text. The specifics of these linkages result in the formation of coiled linear regions, as you can see in this picture of amylose.

Now, it just so happens that iodine ions (I5- and I3- ions in particular) fit very nicely into the core of this helical structure, stabilizing the shape by making contacts with the sugar monomers. The specifics of this interaction are not completely understood, but it seems likely that the blue color results from changes in the electron orbitals of the monomers and iodine ions when this occurs.
Iodine will also react with amylopectin, but with a purple color rather than blue. This color change probably results from a similar interaction, modified by the branched nature of the amylopectin molecule.
As I suggested, research is still being carried out into the interaction between amylose and iodine. If this topic interests you, you may someday be the one to develop a theory that completely explains this interaction!
Here are some references from the literature that may be of interest to you.
Nimz O, Gessler K, Uson I, Laettig S, Welfle H, Sheldrick GM, Saenger W. X-ray structure of the cyclomaltohexaicosaose triiodide inclusion complex provides a model for amylose-iodine at atomic resolution. Carbohydrate Research 2003 Apr 22;338(9):977-86.
Konishi T, Tanaka W, Kawai T, Fujikawa T. Iodine L-edge XAFS study of linear polyiodide chains in amylose and alpha-cyclodextrin. Journal of Synchrotron Radiation 2001 Mar 1;8(Pt 2):737-9.
Good luck on your project!
Steve Mack
Try the links in the MadSci Library for more information on Biochemistry.