Date: Mon Dec 1 07:31:30 2003
Posted By: Dr. James Kranz, Research Scientist
Area of science: Biochemistry
ID: 1069780324.Bc
Message:
Question: what happens to the hydrogen during protein synthesis?
From: Brunetta
Grade: undergrad
City: No city entered., State/Prov.: No state entered. Country: UK
Area: Biochemistry Message ID Number: 1069780324.Bc
what happens to the hydrogen (which helps to form bonds between
base pairs), when the bonds are broken for transcription (during
protein synthesis).
Hi Brunetta,
The process of transcription involves the copying of a DNA sequence into
RNA by RNA polymerase, during which the double-stranded DNA must be
separated so that the polymerase can identify and select the appropriate
RNA building block to add in the growing chain. Translation is the process
of protein synthesis where information encoded in mRNA is translated into
an amino acid code by the ribosome, the chemistry of which involves
transferring individual amino acids from tRNAs into a growing chain of
amino acids called a polypeptide chain. I read your question a couple
times and am not completely sure what you're studying at the moment,
transcription or translation, and whether we're talking about the hydrogen
bonds that hold individual base pairs together in DNA and RNA, or whether
we're talking about the balance of hydrogen atoms in the chemical reaction
that occurs in the ribosome, joining single amino acids together. So I
answered both questions. :)
Hydrogen bonding and Transcription:
As I mentioned, the process of transcription involves the copying
(transcribing) of genetic information encoded in double-stranded DNA into
RNA. For a discussion of the differences between DNA and RNA, you should
refer to a question posed by someone already to the madsci.org website:
How
is DNA better suited than RNA to carry genetic info?
The actual process of copying the DNA sequence into RNA requires that the
double-stranded DNA be first separated into the single DNA strands. One of
the strands will be used as a template from which RNA will be synthesized,
and the other DNA strand just hangs around until the job is done. Next,
the new RNA building blocks, the individual ribonucleotides, form a single
base-pairing interaction with the DNA strand before being attached to the
growing RNA chain. In this way, each A, T(U), G, and C gets added in the
proper order; the RNA that gets made is an exact mirror copy (like a
negative image) of the DNA strand being used as a template, and is
identical in sequence to the other strand of DNA.
Whether we're talking about simple organisms like bacteria, or complex
species like mammals, the basics of transcription are the same. The
transcription reaction is catalyzed by an RNA polymerase (so named because
it makes a polymer of individual RNA nucleotides). Depending again on the
complexity of the organism, this can be a single protein (as is the case in
viruses and bacteria) or can be a mix of multiple different proteins each
carrying out a separate part of the total transcription reaction. (For
more details, feel free to refer to the textbooks at the bottom).
I casually mentioned that the first part of the process involves unwinding
of the double-stranded DNA, the breaking of hydrogen-bonds that hold the
individual base-pairs together. Hydrogen bonds are best described as a
loose interaction between a hydrogen atom on one molecule and a lone pair
of electrons on another molecule (generally we're talking about lone pair
electrons on oxygen or nitrogen). Hydrogen-bonds are non-covalent, which
means they are low energy and transient, similar to the ionic interactions
that exist between common ionic salts. You'll recall from your basic
chemistry books that a single positively charged cation (like Na+) will
associate with a single positively charged anion (like Cl-), and that these
interactions are easily broken (like when you add NaCl to water and the
crystals rapidly dissolve. In much the same way, hydrogen bonds are
readily formed and broken; this is most apparent in a simple solution of
H2O. The structure of H2O has two sets of lone-pair electrons, each
capable of acting as a "hydrogen-bond acceptor" with another H2O molecule.
The tetrahedral geometry of the two lone pair electrons and two hydrogen
atoms around the oxygen are also maintained when two H2O molecules are
H-bonded (in other words, the two oxygen atoms, the hydrogen and the lone
pair electrons are all in a linear orientation). In this rough schematic
(you'll have to imagine what the proper tetrahedral geometry looks like),
the molecule on the left is the "hydrogen-bond donor" and the one on the
right is the H-bond acceptor:
.. ..
:O - H - - :O - H
| |
H H
The curious thing about H-bonds is that the hydrogen atom actually tends to
wander towards the neighboring molecule, becoming partially shared between
the two water molecules in this example. (In technical terms, the hydrogen
is partially abstracted from one of the molecule). So the reality is
closer to this equilibrium, with the hydrogen living somewhere in the middle:
O - H - - O - H < == > O(-) - - H - O(+) - H
| | | |
H H H H
I want to emphasize that, though I've left off the lone pair electrons to
simplify things, they are still present on both molecules; the result of
transferring one hydrogen means the winner takes on a positive charge and
the loser here takes on a negative charge. In reality, the equilibrium is
shifted towards the left (the hydrogen mostly sits on its original oxygen
molecule) and has a partial tendency to shift to the neighboring molecule.
This partial shared character gives the H-bond interaction its strength
and a defined orientation and distance between the two molecules.
Put another way, the reaction in equilibrium is: 2(H2O) < == >
OH- + H3O+
This is basic acid-base chemistry of water; the consequence for biological
reactions is that anytime a reaction mechanism needs either a H+ or a OH-
added or subtracted, the fact that the reactions occur fully surrounded by
water provides an almost infinite source, or a sink, for extra hydrogens
and hydronium ions.
The relevance to DNA, RNA, and transcription is that the two strands of DNA
are held together by hydrogen bonding interactions between opposing
strands. Note that every hydrogen atom that is attached to an oxygen or a
nitrogen is capable of forming a hydrogen bond, and generally does so very
readily with water. When two DNA strands come apart each nucleotide base
is more than happy to satisfy its hydrogen bonding interactions with bulk
water. The extra energy that is gained in a double-helix structure (from
base stacking interactions) favors the double-stranded form of the DNA over
that in water. In terms of hydrogen bonding interactions, they are
satisfied in both the single stranded form (by H-bonding with water) and in
the double-stranded form (by H-bonding with the other DNA strand). The
same is true of RNA, though the structure of RNA is a bit more complicated.
Translation and the mechanism of peptide-bond formation:
I also thought your question might be concerned with the process of
ribosome-mediated translation of mRNA and the chemistry associated with
attaching one amino acid to another. The answer to this question is
relatively straight forward, but the effort required by researchers to
arrive at the answer is truly staggering; that's why I've decided to give
you a brief historical perspective on the study of peptide bond formation
and ribosome function before giving you the punch line.
Understanding the mechanism by which peptide bonds are formed in living
organisms is one of the broadest and most storied histories of modern
biomedical research. The basic reaction of linking one amino acid to
another is referred to as peptide bond formation:
R1 R2 R1 R2
| | | |
- N - C - C = 0 + H - N - C - C = O => - N - C - C - N - C - C = O + H2O
| | | | | || | |
H OH H OH H O H OH
The net balance yields a single bond between the two amino acids plus one
molecule of water, with the -OH coming from the carboxyl group of the first
amino acid and the second hydrogen coming from the nitrogen of the other
amino acid.
It has been known for almost 40 years that the chemical activity giving
rise to peptide bond formation during messenger RNA (mRNA)-directed protein
synthesis can be attributed to the large ribosomal subunit. It has been
known for even longer that the ribosome is comprised of both protein and
RNA components. (In bacteria, the large ribosomal subunit contains ~35
different proteins and two RNAs; mammalian ribosomes are bigger and even
more complex). The amino acids are carried into the ribosome covalently
attached to tRNA; each tRNA associates with the mRNA (using three RNA bases
per amino acid), with each tRNA adding another single amino acid to the
growing chain of new protein. So the equilibrium above is a
simplification; the amino acids each are already covalently attached to
their own tRNA molecule:
R1 R2 R1 R2
| | | |
- N - C - C = 0 + H - N - C - C = O => - N - C - C - N - C - C = O + OH
| | | | | || | | |
H O H OH H O H OH tRNA1
| | |
tRNA1 tRNA2 tRNA2
Note that the balance of the ribosome mediated reaction is different from
that of the previoius schematic; the hydrogen from the NH2 of the second
amino acid gets transferred to the tRNA which leaves with the extra oxygen
from the first reaction. (This is shown in detail in the figure below).
The tRNA coming into the ribosome in the first cycle of the reaction
becomes the carrier of the growing peptide chain in the next cycle of the
reaction. Understanding which of the macromolecular components of the
large ribosomal subunit contribute to its peptidyl transferase site (where
the next amino acid is added in the process), where is that site located on
the ribosome, and how the whole process works has taken a much longer time
to develop.
By 1980, the mystery been reduced to about a half dozen proteins and the
23S rRNA as being the possible catalytic center for ribosome function. In
the early 80's two groups (researchers in the lab of Dr. Thomas R. Cech at
the University of Colorado and the lab of Dr. Norman R. Pace at the
University of Indiana) made the remarkable discovery of catalytic active
RNA in two different types of systems; catalytic RNA is the term given to
RNA that has enzymatic activity, carrying out a bond cleavage reaction
without the assistance of proteins in . At the time, this was a
revolutionary discovery (for which Dr. Cech, but not Dr. Pace,
unfortunately, received the Nobel Prize in Chemistry in 1988). These were
RNA molecules that could break bonds, and it wasn't long before the idea
was revived that 23S rRNA might be the functional component of the
ribosome, where RNA would be the molecule
In 1984, Dr. Harry F. Noller and colleagues at U.C Santa Cruz, using an
RNA-labeling experiment, showed that a highly conserved region (domain V)
in the center of 23S rRNA was intimately involved in the peptidyl
transferase activity. This was also supported by the observation that
mutations in that RNA loop in living E. coli cells renders them resistant
to many inhibitors of peptidyl transferase activity. The evidence
implicating the 23S rRNA as being the catalytic center of the ribosome
continued to mount.
In 2000, the crystal structure was solved of the complete ribosome from an
archaebacterial organism by the group of Dr. Thomas A. Steitz at Yale,
offering the first atomic level view of a functional ribosome. The two
seminal publications for which Dr. Steitz will likely receive the Nobel
Prize some day were published in the journal "Science":
1) Nenad Ban, Poul Nissen, Jeffrey Hansen, Peter B. Moore,
Thomas A. Steitz, Science.(2000) v.289(5481):905-20.
"The Complete Atomic Structure of the Large Ribosomal
Subunit at 2.4 Å Resolution "
2) Poul Nissen, Jeffrey Hansen, Nenad Ban, Peter B. Moore,
Thomas A. Steitz, Science.(2000) v.289(5481):920-30.
"The structural basis of ribosome activity in peptide
bond synthesis."
In the second of these two papers, the detailed catalytic mechanism of
RNA-mediated peptidyl bond formation is finally elucidated. (Incidentally,
the process of determining atomic level structures by crystallography is
EXTREMELY difficult; the Herculean effort required to yield the first real
structural detail of a functional ribosome can be literally counted in
hundreds of man-years within Dr. Steitz's group alone, in addition to the
efforts of scores of other scientists in addition to the ones I've already
mentioned). The reaction is indeed catalyzed by the RNA component of the
ribosome:
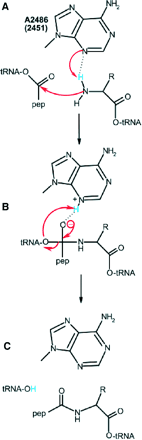
So how does the ribosome actualy perform the chemical reaction? In the
mechansim proposed by Dr. Steitz's group, one of the nucleotides on the big
rRNA, A2486, is thought to have an equilibrium between an unprotonated N3
and a neutral protonated N3. If you take a look at the figure I've uploaded
along with this response, shown in panel (A) of the figure taken from the
Nissen article [Science.(2000) v.289(5481):920-30], "the N3 of A2486
abstracts a proton from the -NH2 group as the latter attacks the carbonyl
carbon of the peptidyl-tRNA". In panel (B), "the protonated N3 stabilizes
the tetrahedral carbon intermediate through hydrogen bonding to the
oxyanion". Finally, in panel (C) "the proton is transferred from the N3 to
the peptidyl tRNA 3' OH as the newly formed peptide deacylates." The
balance is no net change in the number of hydrogens, just a rearrangement
of bonds. The driving force for the reaction comes from another source
(hydrolysis of ATP), which is a topic for another day. Again, we see an
important role of hydrogen bonding in carrying out these rather elegant
chemical reactions.
For additional reading on either transcription or translation, check out
the following textbooks, or refer to any introductory level biochemistry or
molecular biology textbook:
"Biochemistry" 4th ed., Lubert Stryer, New York: W.H. Freeman,
QP514.2.S66 1995
"Genes VII", Benjamin Lewin, New York: Oxford University Press,
QH430.L487 1999
Thanks for your interesting question; I hope I came up with the one of the
answers you were looking for.
Sincerely,
Dr. James Kranz
3D Pharmaceuticals, Johnson & Johnson
Current Queue |
Current Queue for Biochemistry |
Biochemistry archives
Try the links in the MadSci Library for more information on Biochemistry.
MadSci Home | Information |
Search |
Random Knowledge Generator |
MadSci Archives |
Mad Library | MAD Labs |
MAD FAQs |
Ask a ? |
Join Us! |
Help Support MadSci
MadSci Network,
webadmin@www.madsci.org
© 1995-2003. All rights reserved.
