| MadSci Network: Chemistry |
Thanks for the question, Ryan.
You’re right about the spaces in paper towels helping to hold water. The forces responsible for holding the water are called capillary forces are due in part to the surface tension of the water.
You are also right about water being attracted to the cellulose fibers. It is because of this attractiveness that cellulose is termed “hydrophilic.” The reason cellulose fibers are attracted to water is explained by looking at cellulose from a molecular level.
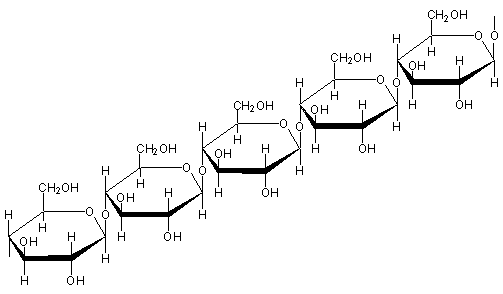
In the attached image, you can see that cellulose is a polymer made of glucose units. The OH groups on the glucose units are what make the cellulose attractive to water. The OH units are polar and since water is also polar, they are attracted to each other.

I hope this answers your question satisfactorily. If you need additional detail, let me know.
Try the links in the MadSci Library for more information on Chemistry.