| MadSci Network: Chemistry |
Dear Mark:
I have a partial answer to your question as to why the solubility of calcium acetate in aqueous solution decreases as the temperature of the solution increases. The complete answer requires the value of a particular number, namely the entropy of solid calcium acetate, which I have not yet been able to find in the literature (I will keep trying, however).
First of all, the solubility of a substance (the solute) can be defined as its concentration in a saturated solution. From a thermodynamic perspective, the extent to which a solute dissolves is governed by two thermodynamic quantities: the “enthalpy change of solution” (often called the “heat of solution”) and the “entropy change of solution” (often called the “entropy of solution”). The symbol for the former quantity is ΔH, and for the latter is ΔS (change in enthalpy and change in entropy).
Let’s start by discussing ΔS. There is a “natural tendency” for many substances to mix when brought into contact in an appropriate manner. For example, if I pour salt onto sand, and I stir or shake the system, the salt and sand will spontaneously form a (heterogeneous) mixture. Similarly, if I add solid calcium acetate (the solute) to water (the solvent) and stir, they will spontaneously form a (homogeneous) mixture, commonly called a solution. In general, when two substances become part of a solution, they are often more randomly dispersed when compared to the separate substances prior to mixing. This natural tendency toward “randomness” is expressed by ΔS. The more random the dispersal, the more positive the corresponding ΔS. However, in the case of an aqueous solution of an ionic compound (such as aqueous calcium acetate), the positive and negative ions attract water molecules to each other. If the attraction is “strong enough” it may be the case that the dispersal of the water molecules could be less random than they were in the solvent form (i.e., the water molecules are “held in place” and not as free to roam as they would be in their solvent form). This means that the actual ΔS could end up being negative (less randomness), instead of positive (more randomness). If that is the case, the extent of mixing will be less favored, and the solubility would be less than otherwise.
The other property to consider is ΔH. ΔH is related to the change in energy that occurs when solute is mixed with solvent. There are many spontaneous processes that in which ΔH is negative. In those cases, the solution is considered to be “more stable” than the separated solute and solvent. In this case the temperature of the solution will rise, which can be interpreted as heat’s being “released” into the environment (an exothermic process). There are, however, spontaneous processes that have a positive value of ΔH (which manifests itself as the cooling of a solution when solute and solvent are mixed, such as the mixing of ammonium nitrate and water).
As a result, the extent to which a solute dissolves in a solvent before the solution becomes saturated can be considered to be the result of a “compromise” between the ΔS effect (tendency to randomness) and the ΔH effect (tendency for stability). The compromise can be expressed quantatively by the expression ΔH − TΔS, where T is the Kelvin temperature of the solution. This combination of terms is given the special symbol ΔG (that is to say, ΔG = ΔH − TΔS), which is called the “gibbs energy” or the “gibbs free energy” of solution. The theory of thermodynamics tells us that if ΔG is highly negative, the process is very spontaneous, and the solubility of the solute will be relatively high. On the other hand, if ΔG is very positive, the solubility will be very low (in extreme cases, the solute will be “insoluble”).
Here’s how the theory might be applied to the solubility values of calcium acetate: At room temperature (around 298 K), ΔG for the process Ca(C2H3O2)2 (s) ---> Ca2+(aq) + 2 C2H3O2−(aq) is negative, and the solid readily dissolves in water and is highly soluble. However, if ΔS for the process is negative, then as T increases, ΔG = ΔH −TΔS will become less and less negative (or more and more positive). This means that the extent to which the solute dissolves in the solvent will become less and less, i.e., its solubility will decrease (the process of dissoving becomes less spontaneous). One can usually determine the ΔS, the ΔH, and the ΔG of various processes from published tables in reference works (such as the CRC Handbook of Chemistry and Physics). I tried to do this for the case of calcium acetate (I searched several such handbooks, and did an extensive Google™ search, but came up empty-handed in the case of finding ΔS (or ΔG). I did calculate (from tables) that for the dissolving of calcium acetate in water, ΔH = +35 kJ/mol (approximately), which means that (if the calculation is correct) adding calcium acetate to water should result in the solution’s cooling (the corresponding value for ammonium nitrate is ΔH = +25 kJ/mol). See if that is the case (and let me know)!
So - - if you can find the numerical value of ΔS, you will be able to get a quantitative estimate of the effect of temperature on the solubility of calcium acetate in water.
Sincerely
Peter M. Fichte
Professor of Chemistry
Coker College
Insert:
Solvation of Ions
Reactions which involve the formation of charged atoms and molecules are
usually extremely endothermic in the gas phase, but may become spontaneous
in certain solvents. If ions are formed from a neutral compound, as when
NaCl is dissolved in water, the oppositely charged cations and anions
naturally attract each other, so formation of a dispersed homogeneous
solution might appear to be energetically unfavorable. To achieve charge
separation of ions in solution, two solvent characteristics are
particularly important. The first is the ability of solvent molecules to
orient themselves between ions so as to attenuate the electrostatic force
one ion exerts on the other. This characteristic is a function of the
polarity of the solvent. Solvent polarity has been defined and measured in
several different ways, one of the most common being the dielectric
constant, ε. High dielectric constant solvents such as water (ε=80),
dimethyl sulfoxide (ε=48) & N,N-dimethylformamide (ε=39), usually have
polar functional groups, and often high dipole moments. When subject to
the electric field of an ion, such polar molecules orient themselves to
oppose the field, and in so doing they limit its reach. Because of
electrostatic attraction between these polar groups, the boiling points of
these solvents are generally higher than those of similarly sized nonpolar
solvents, such as diethyl ether (ε=4.3) and hexane (ε=1.9).
Solvents that have relatively acidic hydrogen atoms (e.g. O-H & N-H) are called protic. Because their functional groups are made up of polar covalent bonds, protic solvents are often polar as well. A list of common protic and aprotic solvents is provided here. The dielectric constants provide a measure of solvent polarity.
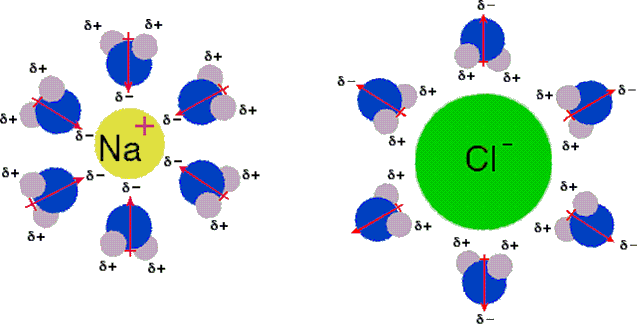
The second factor important in the stabilization of ions, which also resists their intimate recombination, is called solvation. This refers to the ability of solvent molecules to stabilize ions by encasing them in a sheath of weakly bonded solvent molecules, thus somewhat dispersing the electrical charge. Anions are best solvated by hydrogen-bonding solvents; cations are generally solvated by binding to nucleophilic sites on a solvent molecule Two dimensional diagrams illustrating the primary solvation shell about Na(+) and Cl(–) are shown here. The water dipoles are drawn as red arrows, and partial charges are noted. Additional water molecules are oriented in secondary and tertiary layers about the ions.

From this description of ion formation in solution, it should be clear that both enthalpy and entropy factors will be important to the outcome of an ionization process. Thus solvation stabilizes and insulates an ion, helping the enthalpic change, whereas the same solvation adds order and structure to the ionic species at the cost of lowering entropy. The outcome of these interactions is discussed below for two typical salts.
NaCl + H2O ---> Na(+) + Cl(-)
ΔHº = +1.3 kcal/mole ΔSº = +10.3 cal/cal/ ºK mole ΔGº = –1.3 kcal/mole
CaF2 + H2O Ca(2+) + 2 F(-)
ΔHº = +1.5 kcal/mole ΔSº = –36.3 cal/cal/ ºK mole ΔGº = +12.3 kcal/mole
Although these two inorganic salts have similar standard enthalpies of solution in water, their standard entropies are quite different. One might expect this entropy change to be positive, since a single molecule in the solid state produces two or more ionic species, accompanied by an increase in system disorder. However this argument fails to consider the ordering of solvent molecules taking place in the solvation of these ions. Because of their greater charge density, small ions and highly charged ions, such as F– and Ca2+, require greater solvation than large or singly charged ions, such as Na+ or Cl–. The overall entropy change for solution of NaCl is positive, reflecting the increased disorder of ionization, but the entropy change for CaF2 solution is strongly negative thanks to the solvation shell structure required by the resulting ions. These different entropy changes are incorporated in the free energy of solution, which is exergonic for NaCl, but endergonic for CaF2. The result is dramatic. Sodium chloride is quite soluble in water at room temperature (36g per 100g water), but calcium fluoride is nearly insoluble (0.0016g per 100g water).
Try the links in the MadSci Library for more information on Chemistry.