| MadSci Network: Biochemistry |
It's true that saturated fats are "stuffed" with hydrogen and that unsaturated fats are not.
Hydrogenation is used to add hydrogens to unsaturated fats and make them more solid. If a fat is FULLY saturated, it becomes solid, like candle wax. If it is PARTIALLY saturated (the same as partially hydrogenated) the result is a semi-solid, like margarine.
The health problems associated with hydrogenation are really all about partial hydrogenation and cis versus trans fats (after all, who wants to eat candle wax? :)
Trans fat comes from the partial hydrogentation of fats and, except for dairy products, are not naturally found in foods. Trans fats are unsaturated. They are almost (but not quite) stuffed with hydrogen. Trans fats are considerd "unhealthy" because they have been shown to increase cholesterol levels and some scientists believe that it may also increase that risk of cancer.
One possible reason for the problems caused by trans-unsaturated fat is that these fats are not the same shape as the ones our bodies are accustomed to using (saturated or cis-unsaturated fats).
What is the differnce in shape between saturated, cis-unsaturated, and trans-unsaturated fats? I'm glad you asked! Completely saturated/hydrogenated fats are basically a straight chain of carbons, like this...
C-C-C-C-C-C-C-C-C-C-C-C-C-
You can imagine that saturated fats fit together easily because they're flat. The fact that they fit together so easily is one reason that they are solid at room temperature.

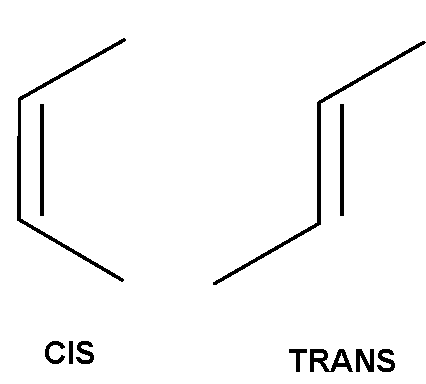
Now for the shape of cis and trans fats. Please see the images (above) I've attached of cis and trans fat. The first thing you might notice is that cis and trans fats have a bend in them. The cis fat looks almost like a C (that's a good way to remember it) and the trans fat looks something like an S. Please keep in mind that I'm showing only the part of the fat that is "bent" into a C or S shape. The rest of the fat molecule is straight.
Now, imagine stacking a bunch of C shapes together. That works, right? What would happen if you tried to put an S in between a couple of C's or an S in between straight chain fatty acids. It wouldn't work so well, would it? In the same way, S-shaped trans fats might not fit into our cells very well.
O.K., I know that this has been a really long answer, but I really want you to understand. To answer your original questions, trans fats may be unhealthy partly because of their unnatural shape. Second, trans fats are produced when liquid vegetable oil is partially hydrogenated.
I hope that this helps!!
Try the links in the MadSci Library for more information on Biochemistry.