| MadSci Network: Chemistry |
I put a penny in vinegar. It came clean. I added a nickel and a dime along with the penny. The nickel and dime are now turning copper. Why does this happen? I am a 8th grade teacher, and all the kids are asking me. I also have a penny in bleach. It did not do anything for a week. Now the bleach is all black and the penny is collecting a greenish-white substance. What is happening chemically in both these cases?
First, what is the composition of pennies, nickels, and dimes?
At one time a penny was made from copper, but that got too expensive so now they are made from an alloy that is 99.8% zinc and 0.2% copper that is plated with pure copper. (See History of Pennies
The five-cent coin was originally a half-dime and made from the same alloy as the dime (see below). After the Civil War its composition was changed to 75% copper and 25% nickel. This alloy is nickel colored, hence the name. http://en.wikipedia.org/wiki/Nickel_(United_States_coin)
The dime and quarter and half-dollar were historically made of an alloy containing 89% silver and 11% copper. [Pure silver was too soft.] They are presently made from silver clad cupronickel. Cupronickel is 92% copper and 8% nickel. http://en.wikipedia.org/wiki/Dime_(United_States_coin)
The penny question – Pure copper is bright copper colored. When copper is exposed to the atmosphere its surface is converted to a dull colored copper oxide. This patina is prized in many architectural situations and bronze statues. When the dull penny is immersed in vinegar (acetic acid) the copper oxide goes into solution as copper acetate (a salt) and exposes a fresh copper metal surface.
CuO + 2CH3COOH = Cu(CH3COO)2 + H2O
Patina acetic acid copper
acetate
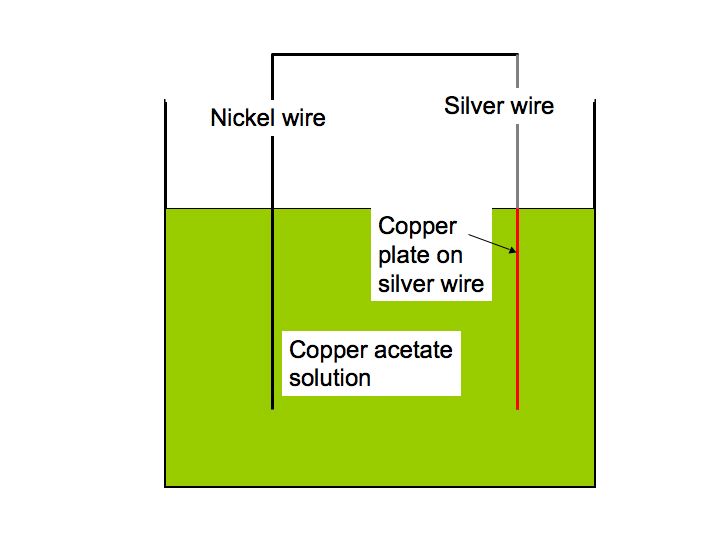
The nickel question requires a bit of electrochemistry. Nickel is a more reactive metal than is copper. More reactive means that when you put the nickel coin into the solution containing the copper acetate (a salt) the nickel in it will reduce the copper salt to copper metal (which plates out onto the nickel) and the nickel will go into solution (corrode) as nickel acetate.
Ni + Cu(CH3COO)2 = Ni(CH3COO)2 + Cu
Copper acetate nickel acetate
Your observation about the dime becoming copper colored can be explained with the same chemistry I discussed in the nickel case but the situation is more complicated. The silver cladding complicates the dime example as silver metal is less reactive than copper and would not reduce a copper salt to copper metal. However, if the dime is in electronic contact with the nickel the nickel would dissolve (corrode) and copper from solution would plate onto the silver. Since the dime and nickel were in the solution together it is possible that they were in electronic contact. [The nickel in the cupronickel core might also reduce the copper acetate in the solution and plate it onto the dime.

Pennies are mostly zinc with some copper (see above).
Household bleach is a 6% solution of sodium hypochlorite in water. Elemental chlorine is added to a sodium hydroxide solution to prepare it. It is quite basic and can oxidize both copper and zinc.
The greenish-white material (a gel) is a mixture of zinc hydroxide (white) with some complicated copper hydroxides. The black stuff is a suspension of cupric oxide. http://en.wikipedia.org/wiki/Copper(II)_oxide
A nice clean experiment is to immerse a clean piece of iron or mild steel (a bolt) into a solution of copper nitrate (or other soluble copper salt). The iron would go into solution and copper metal would plate onto the iron.
Fe + Cu(NO3)2 = Fe(NO3)2 + Cu
Try the links in the MadSci Library for more information on Chemistry.