Date: Fri Dec 1 11:09:38 2006
Posted By: Edwin Rydberg, Post-doc/Fellow, structural biology, IRBM
Area of science: Biochemistry
ID: 1162671820.Bc
Message:
Introduction
Phospholipids or glycerophospholipids as they are also called (to denote
the importance of the glycerol backbone moiety) are bipolar (‘chemical
confused’ or ‘solubility-challenged’ could be the politically correct
terms). Their importance in biology is ubiquitous as they form a majority
of the structure of the cellular membranes. The bipolar nature of
phospholipids is crucial for formation of the cellular lipid bilayers and
for creating an appropriate environment for the solubilization of integral
membrane proteins and cofactors while simultaneously interacting with polar
factors either internal or external to the membrane.
General Structure
The general structure of a phospholipid consists of a glycerol phosphate
backbone that is covalently bonded to two fatty acyl tails and a polar head
group.
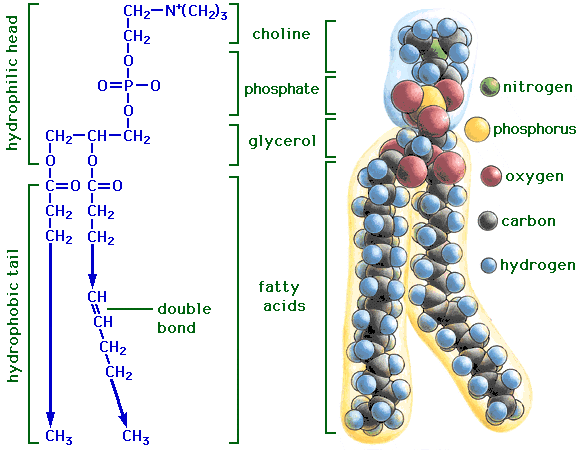 Figure 1: General structure of a phospholipid. (reference:
University of Florida Agricultural and Biological Engineering, Web site of
David P. Chynoweth MSPH, Ph.D
http://faculty.abe.ufl.edu/~chyn/age2062/lect/lect_06/4_18.GIF)
Figure 1: General structure of a phospholipid. (reference:
University of Florida Agricultural and Biological Engineering, Web site of
David P. Chynoweth MSPH, Ph.D
http://faculty.abe.ufl.edu/~chyn/age2062/lect/lect_06/4_18.GIF)
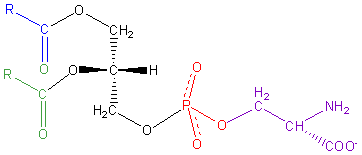 Figure 2: The general structure of Phosphatidyl Choline, showing
the structure of the head group. Other common phospholipids are
Phosphatidyl Serine, Diphosphatidyl-Glycerol, Phosphatidyl Ethanolamine and
Phosphatidyl Inositol.
Both the polar head group and hydrophobic tails vary and result in modified
properties of the bilayer including altered fluidity and/or electronic
properties.
And now…the answer
As I read it, there are two possible interpretations of the questions,
depending on whether ‘it’ refers to the phospholipid or the head group.
However, in both cases the answers are similar in nature, i.e.: there is a
separation of charges, or partial charges.
When we speak of polarity, we are referring to a dispersion of charges or
partial charges (that is: +, -, or neutral). The structure of a
phospholipid (Figure 1) is such that it is composed of a fatty acyl part
and a charged head group (such as highlighted in Figure 2). The fatty acyl
tail is a hydrocarbon and thus uncharged (and hydrophobic) while the head
group is polar (hydrophilic) and consists of either +, - or uncharged,
polar groups such as alcohols. Thus, over the entire phospholipid there is
a polarity, or charge separation, with the uncharged group (fatty acyl
tail) at one end and the polar (head group) at the other.
Likewise, you can see that a similar argument can be applied, albeit on a
smaller scale, to the head group itself (Figure 2). The head group is also
polar due to a dispersion of charges. Here we look at individual atoms to
see that head groups have the basic motif of the lipid, just repeated on a
smaller scale. So we see that there are uncharged hydrocarbon groups
combined with a charged atom (usually nitrogen or oxygen).
A Slightly more chemical explanation
Due to the electronic configuration of carbon (which forms four equivalent
hybrid sp3 valence orbitals) the valence (outer shell) electrons are shared
more evenly between the hydrogen and carbon atoms than is the case with
atoms such as oxygen, nitrogen, or phosphorus. Thus, hydrocarbons are
non-polarized (non-polar, or hydrophobic). When oxygen forms bonds with
hydrogen or carbon, it tends to pull more of the electrons toward itself.
This results in a polarization of the bond as a partial negative charge
forms on the oxygen and a partial positive charge forms on the adjacent atom.
(For references, see any introductory organic chemistry text book. The
concept is also discussed in introductory biochemistry text books, usually
under a section discussing properties of water).
References:
1) Voet & Voet, Biochemistry, Chapter 11: Lipids and Membranes
2) University of Florida Agricultural and Biological Engineering, Web site
of David P. Chynoweth MSPH, Ph.D
http://faculty.abe.ufl.edu/~chyn/age2062/lect/lect_06/4_18.GIF
3) Western Kentucky Biology course site
http://bioweb.wku.edu/courses/BIOL115/Wyatt/Biochem/Lipid/Lipid_2.asp
4) Your favourite organic chemistry text book :)
Figure 2: The general structure of Phosphatidyl Choline, showing
the structure of the head group. Other common phospholipids are
Phosphatidyl Serine, Diphosphatidyl-Glycerol, Phosphatidyl Ethanolamine and
Phosphatidyl Inositol.
Both the polar head group and hydrophobic tails vary and result in modified
properties of the bilayer including altered fluidity and/or electronic
properties.
And now…the answer
As I read it, there are two possible interpretations of the questions,
depending on whether ‘it’ refers to the phospholipid or the head group.
However, in both cases the answers are similar in nature, i.e.: there is a
separation of charges, or partial charges.
When we speak of polarity, we are referring to a dispersion of charges or
partial charges (that is: +, -, or neutral). The structure of a
phospholipid (Figure 1) is such that it is composed of a fatty acyl part
and a charged head group (such as highlighted in Figure 2). The fatty acyl
tail is a hydrocarbon and thus uncharged (and hydrophobic) while the head
group is polar (hydrophilic) and consists of either +, - or uncharged,
polar groups such as alcohols. Thus, over the entire phospholipid there is
a polarity, or charge separation, with the uncharged group (fatty acyl
tail) at one end and the polar (head group) at the other.
Likewise, you can see that a similar argument can be applied, albeit on a
smaller scale, to the head group itself (Figure 2). The head group is also
polar due to a dispersion of charges. Here we look at individual atoms to
see that head groups have the basic motif of the lipid, just repeated on a
smaller scale. So we see that there are uncharged hydrocarbon groups
combined with a charged atom (usually nitrogen or oxygen).
A Slightly more chemical explanation
Due to the electronic configuration of carbon (which forms four equivalent
hybrid sp3 valence orbitals) the valence (outer shell) electrons are shared
more evenly between the hydrogen and carbon atoms than is the case with
atoms such as oxygen, nitrogen, or phosphorus. Thus, hydrocarbons are
non-polarized (non-polar, or hydrophobic). When oxygen forms bonds with
hydrogen or carbon, it tends to pull more of the electrons toward itself.
This results in a polarization of the bond as a partial negative charge
forms on the oxygen and a partial positive charge forms on the adjacent atom.
(For references, see any introductory organic chemistry text book. The
concept is also discussed in introductory biochemistry text books, usually
under a section discussing properties of water).
References:
1) Voet & Voet, Biochemistry, Chapter 11: Lipids and Membranes
2) University of Florida Agricultural and Biological Engineering, Web site
of David P. Chynoweth MSPH, Ph.D
http://faculty.abe.ufl.edu/~chyn/age2062/lect/lect_06/4_18.GIF
3) Western Kentucky Biology course site
http://bioweb.wku.edu/courses/BIOL115/Wyatt/Biochem/Lipid/Lipid_2.asp
4) Your favourite organic chemistry text book :)
Current Queue |
Current Queue for Biochemistry |
Biochemistry archives
Try the links in the MadSci Library for more information on Biochemistry.
MadSci Home | Information |
Search |
Random Knowledge Generator |
MadSci Archives |
Mad Library | MAD Labs |
MAD FAQs |
Ask a ? |
Join Us! |
Help Support MadSci
MadSci Network,
webadmin@madsci.org
© 1995-2006. All rights reserved.
 Figure 1: General structure of a phospholipid. (reference:
University of Florida Agricultural and Biological Engineering, Web site of
David P. Chynoweth MSPH, Ph.D
http://faculty.abe.ufl.edu/~chyn/age2062/lect/lect_06/4_18.GIF)
Figure 1: General structure of a phospholipid. (reference:
University of Florida Agricultural and Biological Engineering, Web site of
David P. Chynoweth MSPH, Ph.D
http://faculty.abe.ufl.edu/~chyn/age2062/lect/lect_06/4_18.GIF)
 Figure 2: The general structure of Phosphatidyl Choline, showing
the structure of the head group. Other common phospholipids are
Phosphatidyl Serine, Diphosphatidyl-Glycerol, Phosphatidyl Ethanolamine and
Phosphatidyl Inositol.
Both the polar head group and hydrophobic tails vary and result in modified
properties of the bilayer including altered fluidity and/or electronic
properties.
And now…the answer
As I read it, there are two possible interpretations of the questions,
depending on whether ‘it’ refers to the phospholipid or the head group.
However, in both cases the answers are similar in nature, i.e.: there is a
separation of charges, or partial charges.
When we speak of polarity, we are referring to a dispersion of charges or
partial charges (that is: +, -, or neutral). The structure of a
phospholipid (Figure 1) is such that it is composed of a fatty acyl part
and a charged head group (such as highlighted in Figure 2). The fatty acyl
tail is a hydrocarbon and thus uncharged (and hydrophobic) while the head
group is polar (hydrophilic) and consists of either +, - or uncharged,
polar groups such as alcohols. Thus, over the entire phospholipid there is
a polarity, or charge separation, with the uncharged group (fatty acyl
tail) at one end and the polar (head group) at the other.
Likewise, you can see that a similar argument can be applied, albeit on a
smaller scale, to the head group itself (Figure 2). The head group is also
polar due to a dispersion of charges. Here we look at individual atoms to
see that head groups have the basic motif of the lipid, just repeated on a
smaller scale. So we see that there are uncharged hydrocarbon groups
combined with a charged atom (usually nitrogen or oxygen).
A Slightly more chemical explanation
Due to the electronic configuration of carbon (which forms four equivalent
hybrid sp3 valence orbitals) the valence (outer shell) electrons are shared
more evenly between the hydrogen and carbon atoms than is the case with
atoms such as oxygen, nitrogen, or phosphorus. Thus, hydrocarbons are
non-polarized (non-polar, or hydrophobic). When oxygen forms bonds with
hydrogen or carbon, it tends to pull more of the electrons toward itself.
This results in a polarization of the bond as a partial negative charge
forms on the oxygen and a partial positive charge forms on the adjacent atom.
(For references, see any introductory organic chemistry text book. The
concept is also discussed in introductory biochemistry text books, usually
under a section discussing properties of water).
References:
1) Voet & Voet, Biochemistry, Chapter 11: Lipids and Membranes
2) University of Florida Agricultural and Biological Engineering, Web site
of David P. Chynoweth MSPH, Ph.D
http://faculty.abe.ufl.edu/~chyn/age2062/lect/lect_06/4_18.GIF
3) Western Kentucky Biology course site
http://bioweb.wku.edu/courses/BIOL115/Wyatt/Biochem/Lipid/Lipid_2.asp
4) Your favourite organic chemistry text book :)
Figure 2: The general structure of Phosphatidyl Choline, showing
the structure of the head group. Other common phospholipids are
Phosphatidyl Serine, Diphosphatidyl-Glycerol, Phosphatidyl Ethanolamine and
Phosphatidyl Inositol.
Both the polar head group and hydrophobic tails vary and result in modified
properties of the bilayer including altered fluidity and/or electronic
properties.
And now…the answer
As I read it, there are two possible interpretations of the questions,
depending on whether ‘it’ refers to the phospholipid or the head group.
However, in both cases the answers are similar in nature, i.e.: there is a
separation of charges, or partial charges.
When we speak of polarity, we are referring to a dispersion of charges or
partial charges (that is: +, -, or neutral). The structure of a
phospholipid (Figure 1) is such that it is composed of a fatty acyl part
and a charged head group (such as highlighted in Figure 2). The fatty acyl
tail is a hydrocarbon and thus uncharged (and hydrophobic) while the head
group is polar (hydrophilic) and consists of either +, - or uncharged,
polar groups such as alcohols. Thus, over the entire phospholipid there is
a polarity, or charge separation, with the uncharged group (fatty acyl
tail) at one end and the polar (head group) at the other.
Likewise, you can see that a similar argument can be applied, albeit on a
smaller scale, to the head group itself (Figure 2). The head group is also
polar due to a dispersion of charges. Here we look at individual atoms to
see that head groups have the basic motif of the lipid, just repeated on a
smaller scale. So we see that there are uncharged hydrocarbon groups
combined with a charged atom (usually nitrogen or oxygen).
A Slightly more chemical explanation
Due to the electronic configuration of carbon (which forms four equivalent
hybrid sp3 valence orbitals) the valence (outer shell) electrons are shared
more evenly between the hydrogen and carbon atoms than is the case with
atoms such as oxygen, nitrogen, or phosphorus. Thus, hydrocarbons are
non-polarized (non-polar, or hydrophobic). When oxygen forms bonds with
hydrogen or carbon, it tends to pull more of the electrons toward itself.
This results in a polarization of the bond as a partial negative charge
forms on the oxygen and a partial positive charge forms on the adjacent atom.
(For references, see any introductory organic chemistry text book. The
concept is also discussed in introductory biochemistry text books, usually
under a section discussing properties of water).
References:
1) Voet & Voet, Biochemistry, Chapter 11: Lipids and Membranes
2) University of Florida Agricultural and Biological Engineering, Web site
of David P. Chynoweth MSPH, Ph.D
http://faculty.abe.ufl.edu/~chyn/age2062/lect/lect_06/4_18.GIF
3) Western Kentucky Biology course site
http://bioweb.wku.edu/courses/BIOL115/Wyatt/Biochem/Lipid/Lipid_2.asp
4) Your favourite organic chemistry text book :)