| MadSci Network: Chemistry |
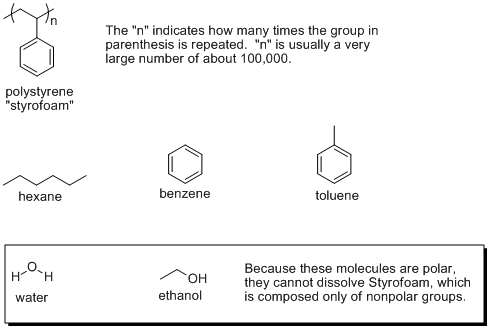
Hello. To answer your question Iíve included a figure with some structures.
Styrofoam does not dissolve readily in hexane. This is because there are benzene side groups coming off of the main molecular chain that interact with each other (a "like dissolves like" type interaction) and do not interact with hexane, which has no benzene like structure. The same is true for water and ethanol.
To get Styrofoam to dissolve, you need a solvent that has benzene as part of the structure. The simplest solvent would be benzene, but others include toluene, which is a benzene molecule with a methyl group bonded to it.
I should note that in Styrofoam (because the benzene groups have strong covalent bonds to the polymer backbone) the benzene groups will not come off (dissociate) when dissolved in any solvent.
Try the links in the MadSci Library for more information on Chemistry.