| MadSci Network: Chemistry |
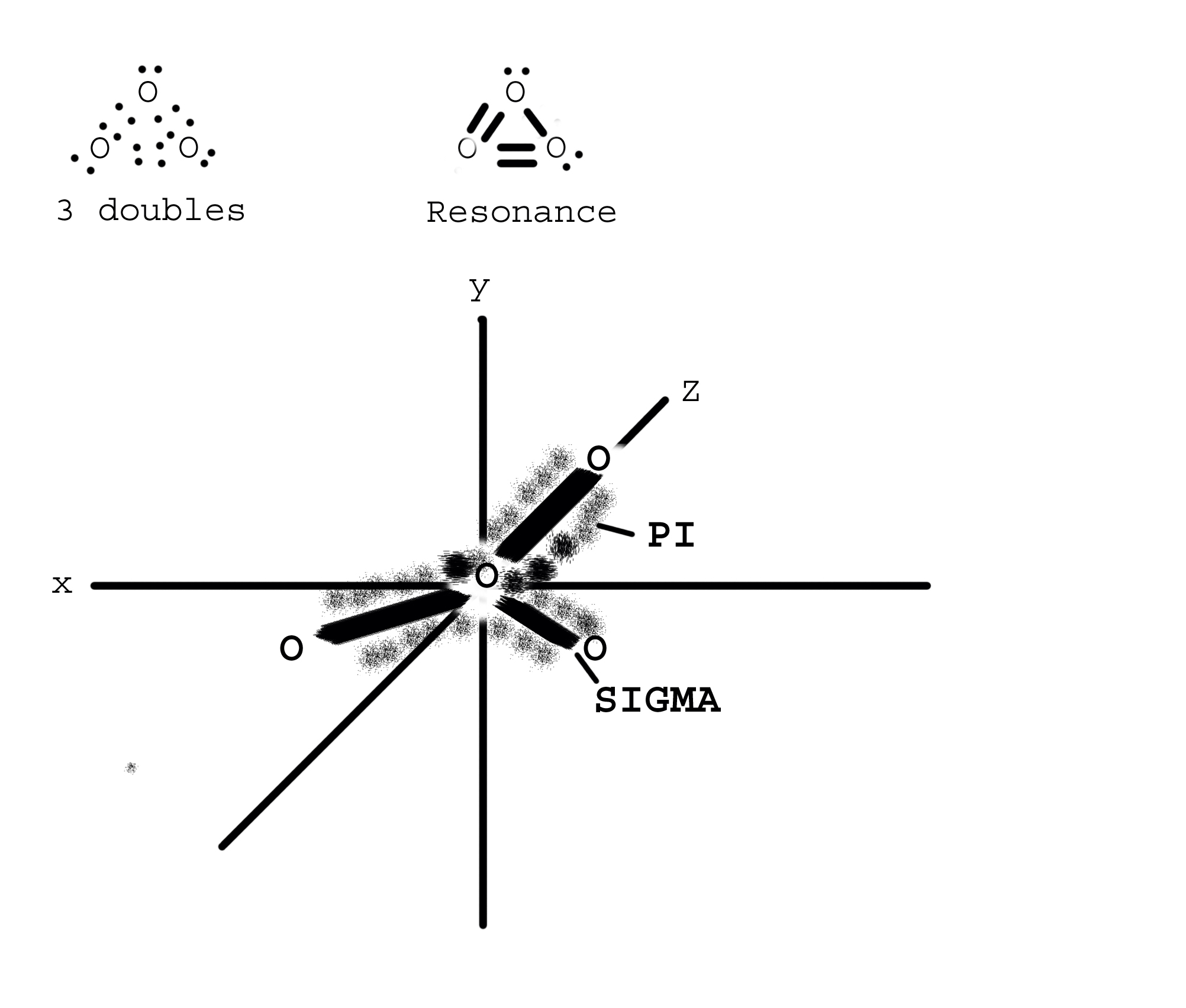
First let me start by stating that I have not taught quantum mechanics in a very long time and I ask that any readers carefully analyze my statements in light of more current research. Part of the problem comes from the way we are being taught bonding. If we think in terms of "electron dot" models of bonding, we are able to put the "dots" wherever we wish. We might try to arrange them in a triangle with double bonds between each atom and the extra electrons distributed around the oxygens. We are further taught to follow the electron pair repulsion concept where all of the pairs repel each other. It would end up looking kind of like an equilateral triangle with 3 double bonds. In this model each atom would have access to 12 electrons. This violates the octete rule. By playing around with various combinations of single and double bonds we can come up with a logical, though incorrect, solution.We can create a structure with some double and some single bonds. We then say that this structure "resonates". That is the locations of the single and double bonds change, through time, always giving each atom access to eight electrons. Seems like a good idea but unfortunately in the early part of the twentieth century our model of the atom changed dramatically. Enter quantum mechanics. The electrons are not, as we might think, able to go wherever we wish them. They are strictly bound by rules generated by energy, relativistic wave properties, charge, and mass. It comes out that electrons must "occupy" distinct regions around the atom. In the lowest possible energy state oxygen atom's outer electrons are in the 2s2 2p4 configuration. This state gives us the classic bent molecule of H20 with single bonds between oxygen and hydrogen. This, however, is not the case with ozone. By allowing the one s electron to gain a bit of energy and 2 p electrons to lose a bit, there appears a new "hybrid" sp2 structure. Although the nature of the bonds is beyond an elementary discussion, let it be said that the sp2 hybrid orbitals line in a flat plane separated by an angle of 120 degrees and that the remaining electrons float in orbitals above and below that plane. Drawing this structure requires a good artist since it must be three dimensional. But let me try to describe it. Each oxygen lies in a flat plane ... let us say the x-z plane for the sake of discussion. There exists a bonding orbital between each atom, referred to as a sigma bond, occupied by 2 electrons each. Above and below this plane is a "y shaped" cloud of electrons referred to as the PI cloud for each atom. These interact to form one complete "y shaped" cloud with some extra PI ears at each atom. The sigma bonds contain 1 electron from each atom. Each atom also has 2 unbonded s orbitals containing 2 electrons each. Therefore each atom has 5 s electrons. The remaining electron resides in the PI cloud. The PI cloud, therefore, has 3 total electrons. The electrons arrange themselves in the cloud in such a manner that the PI clouds are equal. A discerning reader might think that the clouds are unsymmetrical because at any one moment one cloud must have 1 and the other 2 electrons. This is the wonder and mystery of quantum mechanics. In fact each cloud has "sort of" 1 1/2 electrons. Since you can't split an electron, I leave it to the reader to discover how this occurs. E Stammel SUNY Delhi PS: I will post an image on my website at www.delhi.edu [Admin Note: Image is also included below. - RJS]
Try the links in the MadSci Library for more information on Chemistry.