Re: H2O water molecules in a covalent bond repel each other, resulting
Date: Tue Jul 14 13:33:18 1998
Posted By: Dan Berger, Faculty Chemistry/Science, Bluffton College
Area of science: Biochemistry
ID: 891270157.Bc
Message:
891270157.Bc
H2O water molecules in a
covalent bond repel each other, resulting in a distorted tetrahedral bond
of 104.5 degrees. If the bond were to be
linear, how would this arrangement affect life on earth?
As far as I can tell, what you are asking is the effect of water having a
H-O-H bond angle of 180° rather than the actual value of 104.5°. I
can tell you that the effect would be profound.
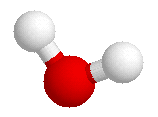 Consider the water molecule as it actually is. The
bonds between oxygen and hydrogen are quite polar. Oxygen holds electrons
more strongly than hydrogen does, and so the oxygen atom accumulates a
negative electrical charge, while the hydrogens have a positive charge.
Consider the water molecule as it actually is. The
bonds between oxygen and hydrogen are quite polar. Oxygen holds electrons
more strongly than hydrogen does, and so the oxygen atom accumulates a
negative electrical charge, while the hydrogens have a positive charge.
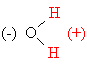 Because the molecule is bent, the hydrogens are both on
the same side of the oxygen atom. This results in one side of every water
molecule having a permanent positive charge, and the other side having a
permanent negative charge; such a situation is called a permanent
dipole. Since opposite charges attract, water molecules are strongly
attracted to each other and to other polar molecules (molecules
having permanent dipoles).
Because the molecule is bent, the hydrogens are both on
the same side of the oxygen atom. This results in one side of every water
molecule having a permanent positive charge, and the other side having a
permanent negative charge; such a situation is called a permanent
dipole. Since opposite charges attract, water molecules are strongly
attracted to each other and to other polar molecules (molecules
having permanent dipoles).
Because the charge separation (or dipole) is so strong in water, it is able
to induce temporary dipoles even in non-polar molecules. The result
is that water is the "universal solvent." Almost anything will dissolve in
water, even if only to a very small extent. Thus, water is able to bring
together a wide variety of substances, which appears to be a prerequisite
for life.
Water molecules attract each other strongly (but not too strongly),
and so water is a liquid over a wide range of temperatures and pressures.
This is important if you want to have liquid solutions of anything...
Even water's high surface tension (the tendency to bead up, rather than
spread out the way alcohol or gasoline does) helps it be uniquely suited
for supporting life -- and water would not have so much surface tension if
it were not such a polar molecule.
Now, if water were linear the O-H bonds would still be polar -- they would
each have a positive and a negative end -- but because they point in
opposite directions the molecule as a whole would be completely
non-polar.
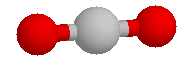 To understand the consequences of this, consider the
similar (but much more massive) molecule carbon dioxide (CO2). Unlike water, carbon dioxide is extremely
reluctant to liquify. CO2 cannot be
liquified at any temperature, if the external pressure is lower than
4.1 atmospheres. The temperature range in which CO2 remains liquid, even at that rather high
pressure, is not large.
To understand the consequences of this, consider the
similar (but much more massive) molecule carbon dioxide (CO2). Unlike water, carbon dioxide is extremely
reluctant to liquify. CO2 cannot be
liquified at any temperature, if the external pressure is lower than
4.1 atmospheres. The temperature range in which CO2 remains liquid, even at that rather high
pressure, is not large.
Furthermore, CO2 is completely non-polar
and so is not able to dissolve very many other substances.
So if water were linear rather than bent,
- it would not liquify except at quite high pressures, and it would
probably not remain liquid over more than about a 20° C. temperature
range at best.
- it would dissolve very few other substances.
In consequence, life as we know it could not exist, not only on earth but
anywhere.
Current Queue |
Current Queue for Biochemistry |
Biochemistry archives
Try the links in the MadSci Library for more information on Biochemistry.
MadSci Home | Information |
Search |
Random Knowledge Generator |
MadSci Archives |
Mad Library | MAD Labs |
MAD FAQs |
Ask a ? |
Join Us! |
Help Support MadSci
MadSci Network,
webadmin@www.madsci.org
© 1995-1998. All rights reserved.
 Consider the water molecule as it actually is. The
bonds between oxygen and hydrogen are quite polar. Oxygen holds electrons
more strongly than hydrogen does, and so the oxygen atom accumulates a
negative electrical charge, while the hydrogens have a positive charge.
Consider the water molecule as it actually is. The
bonds between oxygen and hydrogen are quite polar. Oxygen holds electrons
more strongly than hydrogen does, and so the oxygen atom accumulates a
negative electrical charge, while the hydrogens have a positive charge.
 Because the molecule is bent, the hydrogens are both on
the same side of the oxygen atom. This results in one side of every water
molecule having a permanent positive charge, and the other side having a
permanent negative charge; such a situation is called a permanent
dipole. Since opposite charges attract, water molecules are strongly
attracted to each other and to other polar molecules (molecules
having permanent dipoles).
Because the molecule is bent, the hydrogens are both on
the same side of the oxygen atom. This results in one side of every water
molecule having a permanent positive charge, and the other side having a
permanent negative charge; such a situation is called a permanent
dipole. Since opposite charges attract, water molecules are strongly
attracted to each other and to other polar molecules (molecules
having permanent dipoles).
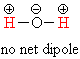

 To understand the consequences of this, consider the
similar (but much more massive) molecule carbon dioxide (CO2). Unlike water, carbon dioxide is extremely
reluctant to liquify. CO2 cannot be
liquified at any temperature, if the external pressure is lower than
4.1 atmospheres. The temperature range in which CO2 remains liquid, even at that rather high
pressure, is not large.
To understand the consequences of this, consider the
similar (but much more massive) molecule carbon dioxide (CO2). Unlike water, carbon dioxide is extremely
reluctant to liquify. CO2 cannot be
liquified at any temperature, if the external pressure is lower than
4.1 atmospheres. The temperature range in which CO2 remains liquid, even at that rather high
pressure, is not large.