| MadSci Network: Chemistry |
Mandy,
Ascorbic acid, or Vitamin C, is oxidized by iodine according to the following equation:
Since this is performed under aqueous conditions, the hydrogen iodide is ionized, and we normally write
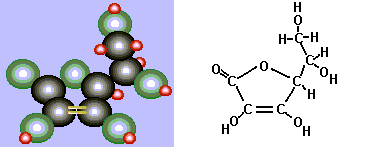
Of course this does not tell the whole story. The structure of ascorbic acid (centered around a five-membered ring of four carbons and one oxygen atom) includes two adjacent alcohol(OH) functional groups.

These two alcohol groups each lose a hydrogen atom to the iodine. The ascorbic acid is thereby oxidized, and the iodine reduced to iodide. Yes, it is a redox reaction! Note that there is a double bond between the two carbon atoms with the alcohol groups attached in the reduced (starting) form. This ends up as a single bond when the alcohol groups are converted into carbonyl groups.
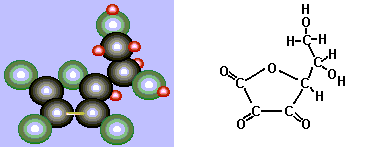
The product of oxidizing ascorbic acid (C6H6O6) is, not surprisingly, named dehydroascorbic acid.
The most common way to determine ascorbic acid with iodine is actually to back- titrate excess I2 with thiosulphate. Can you think why that method might be preferred over straightforward titration with stock iodine solution?
It can also be titrated directly with an indicator (PDF file). /p>
Have you tried a vitual titration? (http://www.intschool-leipzig.com/bailey/tutorial/acidprop/expmtq2.htm link defunct as of 5/2006). It does use an applet and might not run on all browsers. Although it is an acid-base titration and not a redox, it is fun!
Try the links in the MadSci Library for more information on Chemistry.