| MadSci Network: Chemistry |
Daniel,
Benedictís test is an analytical chemical test that reveals the presence
of aldehydes. An aldehyde is an organic compound having the chemical
functional group CHO, which consists of a carbon atom doubly bonded to an
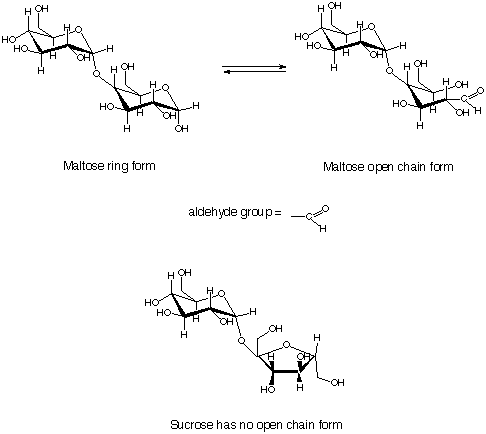
oxygen atom and singly bonded to a hydrogen atom. The graphic that
accompanies this answer shows you what an aldehyde group looks like.
Some sugars exist in solution as an equilibrium mixture of their ring
form and an open chain form. The open chain form contains an aldehyde
group, which is formed when the ring opens. When the ring closes, the
aldehyde group is no longer present. Thus Benedictís test will only give a
positive test for a sugar that is present at least partly as an open chain,
or aldehyde, form. Maltose is, but sucrose is not.

Try the links in the MadSci Library for more information on Chemistry.