| MadSci Network: Chemistry |
What is capillary action?
Capillary action is a physical effect caused by the interactions of a liquid with the walls of a thin tube. The capillary effect is a function of the ability of the liquid to wet a particular material.
The liquid for which this effect is most commonly seen is water, because water is capable of strong surface interactions and because water is ubiquitous.

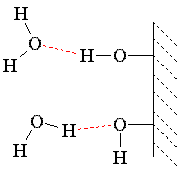
Water climbs up a thin glass tube because of the strong hydrogen-bonding interactions between the water and the oxygens (and terminal hydrogens) at the surface of the glass (SiO2; surface oxygens are typically bonded to hydrogen). The energetic gain from the new intermolecular interactions must be balanced against gravity, which attempts to pull the liquid back down. Therefore, the narrower the tube, the higher the liquid will climb, because a narrow column of liquid weighs less than a thick one.
One gets the same effect for pores and narrow spaces in any hydrophilic object. While I have not been able to find any information on the Web, it is my suspicion that a narrow tube coated with a hydrophobic substance (such as Teflon) should not show a capillary effect.
The obvious question is, "what effect will microgravity have on capillary properties," and in fact a Yahoo search for "capillary action microgravity" gave several hits. Apparently this is a topic currently being thoroughly investigated.
For more information:
| Dan Berger | |
| Bluffton College | |
| http://cs.bluffton.edu/~berger |
Try the links in the MadSci Library for more information on Chemistry.