Suppose we are in the shell n=2 of an atom. It has 2 orbitals, the s-orbital and the p-orbital. The p-orbital has an "8" shape. But, how can an electron go past the n=2 shell to the n=1 shell through the nucleus and back to the n=2 shell? I mean, electrons cannot pass through the space between n=1 and n=2, isnít it? And it canít pass through the nucleus, isnít it??? Please help.
Your question is based on what, in philosophy, is called a "category mistake." Let me explain.
Electrons are particles, fair enough. But de Broglie showed that, under the right conditions, particles have wave properties. That's why electron microscopes work--electrons can be focused just like light waves! And in the very small volume of an atom, the wave properties of electrons dominate.
Atomic orbitals are standing waveforms, like the vibrations of a drumhead. They have positive and negative amplitudes, just like a wave; and they have nodes, where the amplitude goes to zero before changing sign, just like a wave. Here's a beautiful explanation of atomic orbitals in terms of standing waves, based at the University of Idaho.
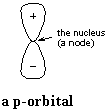 A p-orbital has a node at the nucleus, with one lobe on either side of the
nucleus, as shown at right. Now, like any wave, the amplitude of the
p-orbital goes to zero at the node, as the wave changes sign from plus to
minus, or from minus to plus. We interpret the wave (actually, the
square of the wave so that there are no longer plus and minus signs) as the
probability of finding an electron at any given point.
A p-orbital has a node at the nucleus, with one lobe on either side of the
nucleus, as shown at right. Now, like any wave, the amplitude of the
p-orbital goes to zero at the node, as the wave changes sign from plus to
minus, or from minus to plus. We interpret the wave (actually, the
square of the wave so that there are no longer plus and minus signs) as the
probability of finding an electron at any given point.
- Here's the tough thing to wrap our minds around:
- An electron in an atom is NOT, in any observable sense, "zipping around" in the atom like a fly on amphetamines. (As soon as we concentrate on such particle-like behavior, "atomic orbitals" are no longer valid anyhow.) Instead, it exists as a standing waveform within the atom.
A p-orbital describes the probability of finding a "point-like" electron in the (n=2, l=1) energy level, at any particular place, if we were to (for example) shoot another electron at that location. And it tells us that an electron in a p-orbital has a ZERO chance of being found in the nucleus. (This is not the case for an s-orbital, which has no node at the nucleus.)
Where is the electron when we're not trying to bounce other electrons off of it? "In the atom" is as close as we can come--within the limits imposed by the fact that it is NEVER at a node!
It may be more useful, when considering atomic orbitals, to think of the electron as being "smeared out" over the space defined by the orbital. In fact, chemists and molecular physicists often talk in just that way: rather than speaking of electrons in atoms and molecules, we may speak of "electron density"--the amount of negative charge at any given point (balanced, of course, by the positive charge from nuclei which ARE little point-like objects).
I recognize that my explanation may be confusing or weird or even shocking, but it was the great quantum physicist Neils Bohr who said that anyone who is not shocked by quantum mechanics has not understood it. For another example of the problems that arise when we try to apply ideas about particles to electrons in atoms, see this MadSci answer.
| Dan Berger |
| Bluffton College |
| http://www.bluffton.edu/~bergerd |