| MadSci Network: Chemistry |
Hello, Michael - thanks for the question about "neon" lights. I recently visited Las Vegas and probably saw about half of the neon lights in the world! Lots of different colours.
Anyhow, they are not all "neon" lights. To explain, starting with a little history. In the late 1800ís physicists (notably Sir William Crookes) began experimenting to see what radiation is generated in evacuated tubes to which small amounts of elemental gases were introduced - when an arc was struck between electrodes inside the tube. They found that many different coloured effects were seen depending on what gases were introduced. It wasnít until 1910 that the first application of this to illuminated signs became practical when the French Physicist, Claude Georges developed the first neon light - obviously choosing the rare inert gas, neon as the filling, but also finding the right pressure and voltage to apply to get the best effect - which is a deep red colour. With the increased availability of other rare gases, and experiments on tube and electrode design, soon a variety of differently coloured tubes were used to decorate and advertise products and night scenes in big cities had changed in a kind of gaudy way. The other rare gases - helium, argon, krypton and xenon are used as are sodium vapour and mercury vapour - the latter two being very widely used today in street lighting. All of these kinds of lights are called gas discharge lamps and they work with quite low pressures of the gas or gas mixture in question - (normal pressure gases donít work very well because itís difficult to initiate a discharge - which need quite a high voltage - 5000 to 15000 volts - but not much current). Helium gives a pale yellow colour, while argon gives a nice blue. Most neon signs these days contain a mixture of neon and mercury or just neon alone, and often the colours are actually produced by coating the insides of the tube with material which absorbs the neon or neon-mercury emissions and then fluoresce at a different wavelength. Sometimes, mercury alone is used inside a tube of coloured glass.
So whatís going on? Well the electric voltage discharges in the gas, exciting the gas molecules to a higher energy state (called and excited state) which doesnít last long and collapses back to the normal state emitting its excess energy as light. because the difference in energy between the normal (ground) state and the excited state is fixed for a given gas, you always get a characteristic wavelength of light emitted for that gas. This energy difference is related to the wavelength emitted in that if you multiply the energy by the wavelength, the result equals Planckís constant times the speed of light. In other words, the energy times the wavelength is constant and so high energy means short wavelength (more blue) and low energy means longer wavelength (more red). Now you should be able to tell me which energy difference is greater - ground to excited state of neon or argon??
Just for your interest, many lasers (Light Amplification by Stimulated Emission of Radiation) are based on gas discharge tubes - and often mixtures work best - e.g. helium and neon which synergistically excite each other and helped by mirrors produce very high and coherent light energy which is characteristic of lasers.
Hope this answers your question.
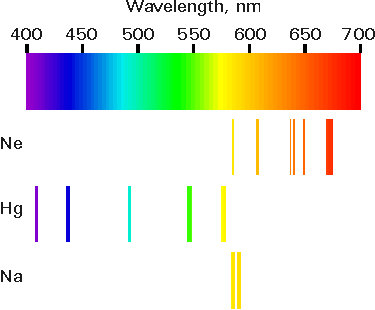
Finally hereís a diagram which shows where in the spectrum some of the gases emit light.

Try the links in the MadSci Library for more information on Chemistry.