Date: Wed Mar 29 10:18:59 2000
Posted By: David Reibstein, Staff, Princeton Materials Institute, Princeton University
Area of science: Chemistry
ID: 953748083.Ch
Message:
alcohol
A quick answer is that alcohol freezes and boils at a lower temperature
than water because its molecules are not held together as tightly as the
molecules of water. Here is an explanation::
Let's start with boiling. When a liquid boils, it changes from
a liquid to a gas. What is the difference between these two states
of matter? In a liquid, the molecules it is made of are pretty close
together. You can think of them as stuck"" together. But in
a gas the molecules are very far apart. So a liquid
doesn't go flying all around like a gas does. A liquid stays
together.
How does the change from liquid to gas occur? It requires energy
(that's why you have to heat it), and this energy makes the molecules move
faster.
As they move faster they bounce off each other more and are able able to
fly
away as a gas. So, alcohol boils at a lower temperature because it
takes less energy to makes the alcohol molecules "unstick" from each other.
Why is this? Why do water molecules stick together more tightly
than alcohol molecules? You probably know that the formula for water
is H2O. That is, 2 hydrogen atoms and 1 oxygen atom.
These atoms are put together like this: H-O-H. Now, it happens
that the H atoms have a positive (+) charge on them, and the oxygen atom
has a negative (-) charge. You probably know that opposite charges
attract. So the H atoms of one water molecule are attracted to the
O atoms of another water molecule, and all of the water molecules are stuck
together, like this:
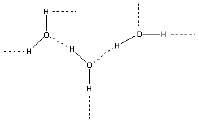 |
Here, the solid lines are the bonds between atoms within the water
molecules, and the dashed lines are the attractions between one water
molecule
and another.
These attractions add up to something very strong, so it is hard to
separate
water molecules from each other and it takes a high temperature to boil
water. |
Alcohol molecules are also held together in a similar way, but the
attractions are weaker. Here's why: The formula for ethyl
alcohol
(the kind that adults drink) is C2H5OH. Notice
that
this formula has OH in it just as in water. So alcohol molecules can
stick
together, too, like this:
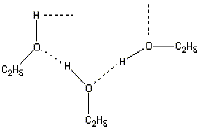 |
But notice that the C2H5 part does
not
have attractions for other molecules. So the alcohol molecules are not
held
together by as many attractions as are the water molecules. So
it
takes less energy to separate or "unstick" alcohol
molecules
from each other, and alcohol boils at a lower temperature. |
What about freezing and melting? The same thing. When a
solid
melts, its molecules go from being held together very tightly to being held
together less tightly. Again, it takes more energy to do this for
water
than for alcohol, so the melting point for water is higher than for
alcohol.
David Reibstein, Outreach Director, Princeton Materials Institute,
Princeton
University
Current Queue |
Current Queue for Chemistry |
Chemistry archives
Try the links in the MadSci Library for more information on Chemistry.
MadSci Home | Information |
Search |
Random Knowledge Generator |
MadSci Archives |
Mad Library | MAD Labs |
MAD FAQs |
Ask a ? |
Join Us! |
Help Support MadSci
MadSci Network,
webadmin@www.madsci.org
© 1995-2000. All rights reserved.

