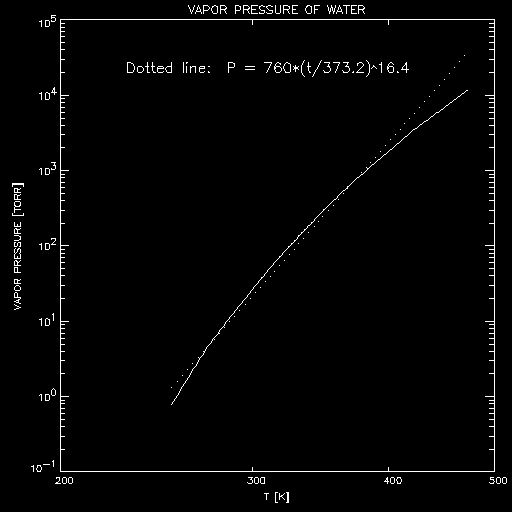
 shows a plot of the vapor pressure
of water, along with an approximation (dotted line) which shows it is
fairly well approximated by a high power
of the absolute temperature K (T [K] = T [C] + 273.2).
shows a plot of the vapor pressure
of water, along with an approximation (dotted line) which shows it is
fairly well approximated by a high power
of the absolute temperature K (T [K] = T [C] + 273.2).
| MadSci Network: Biophysics |
Really neither, unless you want to call "outgassing" a slow explosion. (I should warn you in advance that, since I am not an expert in space medicine, the following is only based on my general knowledge of astronautics and of physics. However I believe I know enough to answer your question, and I hope any inaccuracies are minor.)
Because the human body is something like 85% water, the behavior of
water in space is the key to understanding this subject.
Suppose we fill a big bottle half full of water in a vacuum
-- but let's say not in zero g, on the ground rather --
at some temperature T, and then seal the bottle.
The water will begin to evaporate pretty quickly.
What that means is that some of the water molecules near the
surface of the liquid will simply fly off into the vacuum.
How many molecules do this and how fast they escape depends on T --
there will be more, faster, if it is hot, and fewer, slower if it is cold.
But soon the empty part of the bottle will begin to have quite a few
water molecules in it, until what was vacuum is filled with water vapor.
Then the molecules in the vapor part of the bottle will start hitting the
liquid and sticking, until as there is more and more vapor, at some point
the number of molecules returning to the liquid from the vapor will equal
the number escaping from the liquid into the vapor.
(In order to keep T constant during this process, we will
have to warm the bottle gently, as when water molecules escape from the
liquid
it will cool.)
But eventually we will arrive at a situation in which the temperature is
still T, part of the bottle contains liquid water, and part contains
water vapor (no air, notice, since we started in a vacuum), and no
more water seems to be evaporating from the liquid -- because
in microscopic terms, the net of molecules escaping and returning is zero.
The pressure of the vapor depends on T only, and is
called the vapor pressure p(T) of water at a
temperature T.
The figure  shows a plot of the vapor pressure
of water, along with an approximation (dotted line) which shows it is
fairly well approximated by a high power
of the absolute temperature K (T [K] = T [C] + 273.2).
shows a plot of the vapor pressure
of water, along with an approximation (dotted line) which shows it is
fairly well approximated by a high power
of the absolute temperature K (T [K] = T [C] + 273.2).
What does all this mean for an unprotected person in space? The vapor pressure of water at body temperature, 98.6 F or 37 C, is about 0.93 pounds per square inch (PSI), or 48 mm of mercury (Hg). For comparison, the pressure of the atmosphere is 14.7 PSI or 760 mm Hg. Since the surrounding pressure is zero in space, water in the body might be expected to start boiling furiously, except for one important thing: the water in the body is very significantly contained by skin, blood vessels, etc. If the vapor pressure at body temperature were 1 atmosphere, the results would be immediately disastrous, as the skin and blood vessels cannot contain such a high pressure. The result might well be something like an explosion, certainly severe rupture and hemorrhaging from many points. But since the actual pressure is only about 50 mm, we have to ask how much pressure can the skin and blood vessels really contain? Well, the normal blood pressure in the arteries is 70-150 mm Hg, so there is no way the blood is going to boil in the arterial side of the circulatory system, so long as one has a working heart and normal blood pressure. Furthermore, the arteries can contain at least 200 mm for short periods without rupture. Even the veins have to withstand the pressure differences caused by gravity between head and feet when we stand up, and these must be of the order of 75-150 mm, well above 50 mm.
It appears to me that the main source of water loss will be through the lungs. As soon as one is exposed to the vacuum, almost all of the air in the lungs will escape, since one cannot effectively contain more than a fairly low pressure, much less than 1 atmosphere, by closing the mouth and nose. So taking a deep breath in advance won't help much. After that, the lungs should fill with water vapor at about 50 mm pressure, mixed with some gases that are dissolved in the blood, oxygen and carbon dioxide certainly, and some nitrogen. Because 50 mm is such a low pressure, I believe that a trained person could significantly slow the loss of water vapor and gases from the lungs by simply trying to keep the mouth and nose closed as much as possible. Then unconsciousness and death would come when the amount of oxygen in the blood was sufficiently depleted. The time for this to occur would probably depend on the oxygen content of the blood immediately prior to the depressurization; it could range from a few seconds if you were caught in the midst of strenuous exercise, to possibly as long as a minute or more if one had some warning and were to hyperventilate in advance.
After death, water and gases would continue to escape slowly, and the interior of the body would cool off. The cooling would cause the vapor pressure to drop even more; at freezing temperatures it is less than 5 mm Hg. Thus the escape of water vapor (along with gases and other volatile substances) would be progressively slower and slower. This process is known as "outgassing", and happens whenever anything with a significant content of volatile substances is placed in a high vacuum or in space. In the end, the body would be entirely dried out, dessicated, and reduced to a small fraction of its original mass.
Try the links in the MadSci Library for more information on Biophysics.