| MadSci Network: Chemistry |
how were permanent waves
for hair discovered
what is the history of the development of permanent wave hair products
and the different types of perms available today.
How do they differ from earlier perms.
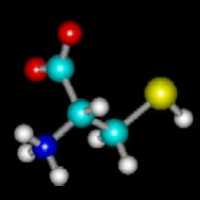 The chemistry of permanent waves is pretty straightforward. Hair
is largely made of keratin, a protein that contains a lot of the amino acid
cystine. The structure of cystine is shown at left. The image is
color-coded:
The chemistry of permanent waves is pretty straightforward. Hair
is largely made of keratin, a protein that contains a lot of the amino acid
cystine. The structure of cystine is shown at left. The image is
color-coded:

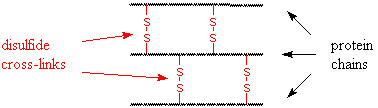
A permanent wave treatment has three steps:
- Break the disulfide bonds with a reducing agent.
- Shape the hair into the desired configuration, usually with curlers; the size of the curler will determine the tightness of the resulting curl.
- Reform the disulfide bonds in new positions, using an oxidizing agent.
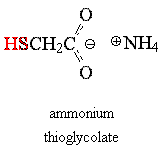 Typically a chemical
base is used as the reducing agent; the Ogilvie® home permanent I checked uses ammonium
hydroxide -- a solution of ammonia, NH3, in
water -- and ammonium thioglycolate as the active ingredients in the
"Waving Lotion." These compounds (the ammonia and the thiol) account for the stench we associate with
permanents.
Typically a chemical
base is used as the reducing agent; the Ogilvie® home permanent I checked uses ammonium
hydroxide -- a solution of ammonia, NH3, in
water -- and ammonium thioglycolate as the active ingredients in the
"Waving Lotion." These compounds (the ammonia and the thiol) account for the stench we associate with
permanents.
The oxidizing agent, in the "Neutralizer," is good old hydrogen peroxide (H2O2), probably at a concentration similar to that in the disinfectant you have in your medicine cabinet.
Lots of other ingredients are listed, but they are all surfactants (soaps and detergents are surfactants; their function is to allow organic molecules to dissolve in water).
I am sorry that I can't say much about the history of permanent waves. The only thing I know about the history is something I picked up from The Autobiography of Malcolm X: in the 1930s and 1940s, black people were using lye (sodium hydroxide) to straighten their hair. My wife also tells me that lye has traditionally been used (as the reducing agent, of course) in home permanents.
For further information, I suggest you look for back issues of Chemical & Engineering News in a local college library.
| Dan Berger | |
| Bluffton College | |
| http://cs.bluffton.edu/~berger |
Try the links in the MadSci Library for more information on Chemistry.