Linus Pauling, in his classic text The Nature of the Chemical Bond, proposed that main-group elements (that is, non-metals) whose valence shells have principal quantum number 3 or higher can use valence d-orbitals to form bonds. Phosphorus, for example, can form PF5.
Compound |
Hybridization |
Valence |
|
| CH3CºP | sp | 8 | |
| R2C=PC(CH3)3 | sp2 | 8 | |
| (CH3)3P | sp3 | 8 | |
| PF5 | sp3d | 10 | |
| SF6 | sp3d2 | 12 |
This model has been disputed, and with good reason: the valence d-orbitals of main-group elements are too high in energy to participate in bonding. See W. Kutzelnigg, Ang. Chem. Int. Ed. Engl. 1984, 23, 272-295, and the references given below.
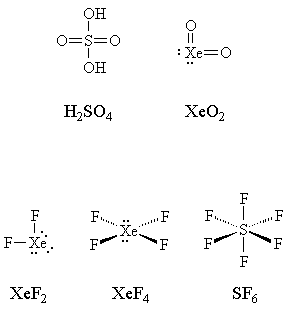 |
We would then see bonding in XeF2 as resulting from sp3d hybridization. d-Orbitals must become involved because there are a total of 10 valence electrons around Xe, which require accommodation. This is the "expanded octet" model and has the advantage of being simple to represent as a Lewis structure (see structures at left). |
Electronegativity comes into play, too. Notice that the elements that form so-called hypervalent compounds only do so with elements which are much more electronegative: typically with fluorine or oxygen, though antimony and tellurium will do so with chlorine. This hints at a way to represent these compounds without "violating the octet rule." For example, we could draw the following Lewis structures for sulfuric acid and for xenon dioxide, which do not violate the octet rule:
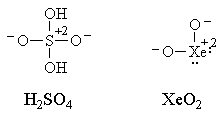
We could even draw unconventional structures (with the bonds shuffled by resonance) for xenon difluoride, xenon tetrafluoride and sulfur hexafluoride which do not violate the octet rule:
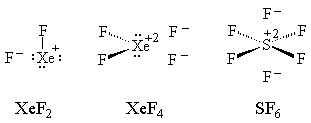
This sort of Lewis structure is strongly supported by quantum chemical calculations: A. Reed and P.v.R. Schleyer, J. Am. Chem. Soc. 1987, 109, 7362-73; ibid. 1990, 112, 1434-45; E. Magnusson, ibid. 7940-51.The qualitative interpretation of this is that the central atoms have a loose enough hold on their valence electrons that other, more strongly electronegative elements can come in and strip some of them away.
But remember, all this is explanation after-the-fact. Chemical compounds form as they will (for example, though argon should be able to participate in hypervalent bonding, no one has ever been able to coax it into a compound; and krypton only forms KrF2 very reluctantly), and we can't always predict how they will do so.