| MadSci Network: Chemistry |
What is the Lewis structure of the ClO- ion and the ClO2- ion?
I am a Biology teacher assigned a chemistry class and I cannot
find the answer to the questions posed above. I have a pretty
good idea about drawing the Lewis structures for both of these
ions, but I don't know how to determine which one has a coordinate
covalent bond.
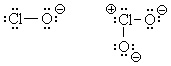 Lewis structures for almost anything can be found by following a few simple
rules. The structures for ClO- (hypochlorous ion) and
ClO2- (clorous ion) are quite
simple and are shown above, left.
Lewis structures for almost anything can be found by following a few simple
rules. The structures for ClO- (hypochlorous ion) and
ClO2- (clorous ion) are quite
simple and are shown above, left.
![]() Notice that these Lewis structures show all atoms with an octet. One could,
I suppose, use an "expanded octet" structure for chlorous ion as
well; such a structure is shown at right.
Notice that these Lewis structures show all atoms with an octet. One could,
I suppose, use an "expanded octet" structure for chlorous ion as
well; such a structure is shown at right.
Nevertheless, the structures which preserve 8-electron octets are more valuable for determining which compound has a "coordinate" covalent bond, because such a bond is defined as one formed from electrons "belonging" to only one of the atoms. Examples of such bonds include ammonium ion (NH4+), hydronium ion (H3O+), and phosphine oxide (H3PO); in all of these, a bond is formed by donation of a pair of electrons to an electron-deficient species (H+ or O). This donation always results in the formal charge on the acceptor atom being reduced by one unit (from +1 to 0, or from 0 to -1).
You will also notice that in every case the electron donor atom is assigned a single formal positive charge for each pair of electrons donated. So the clue to finding coordinate covalent bonds within Lewis structures is to look for formal positive charges: such a charge is found in ClO2-.
For more on this, see one of my recent answers.
| Dan Berger | |
| Bluffton College | |
| http://cs.bluffton.edu/~berger |
Try the links in the MadSci Library for more information on Chemistry.