Date: Fri Oct 5 12:13:36 2001
Posted By: Joseph Weeks, President
Area of science: Chemistry
ID: 992334710.Ch
Message:
Pure elements generally have specific, well defined properties such as
melting points. Pure molecular compounds often also have well defined
melting points. Indeed, the triple point of water (the temperature at
which H2O exists simultaneously as a gas, liquid, and water) is a basis of
calibrating most temperature scales.
Once you get combinations of elements, however, melting points can start
acting funny. Particularly with metals, the elements start to interact
with each other. As long as one metal has some solubility in the other,
you can expect to have changes in melting points compared to what is
observed for pure metals. Sterling silver is a compound consisting of
about 5% copper
in 95% silver, and is a good example of how complicated melting points can
become.
In a mixture of two elements, usually called a binary mixture, the behavior
of the compound as a function of composition and temperature is presented
in a "phase diagram." http://cyberbuzz
.gatech.edu/asm_tms/phase_diagrams/
presents a large number of phase diagrams for different binary combinations
of metals. They present the phase diagram of silver and copper at http
://cyberbuzz.gatech.edu/asm_tms/phase_diagrams/pd/ag_cu.gif .
Attached to this answer is the same phase diagram that I have modified in
order to attempt to answer your question.
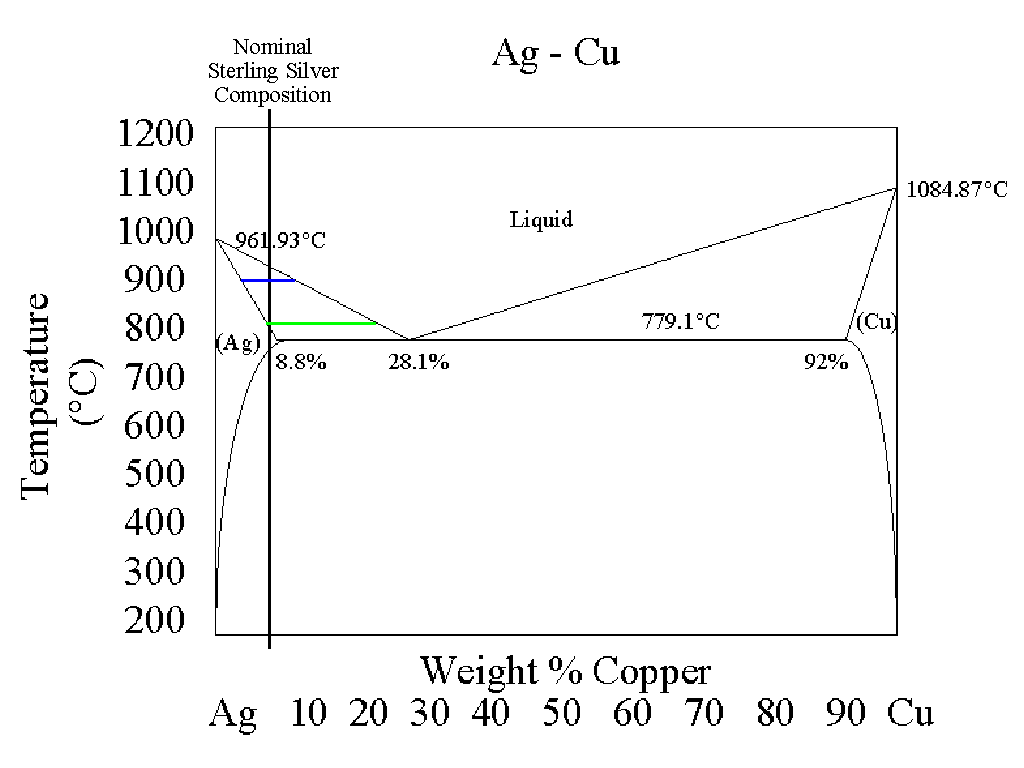 I have drawn a vertical line at the 5% Cu composition. This line represents the
composition of sterling silver.
According to this diagram, as a lump of sterling silver is heated, nothing
much happens until the temperature reaches about 760oC or so. Above that
temperature, the sterling silver starts to melt. The phase diagram shows
us that the composition of the material that first melts contains 28.1%
copper (and 71.9% silver), while that portion of the lump which hasn't
melted is essentially pure silver. As the mixture is heated to about
800oC, the green line shows that the liquid portion of the mixture now has
a composition of roughly 20% copper, with the solid phase still consisting
of essentially pure silver. At 900oC, the liquid phase contains about 8%
copper (as more and more silver has dissolved into the liquid phase) but
still contains some solid silver. Not until the temperature reaches 930 or
940oC has all of the silver dissolved into the liquid phase.
In metal alloys (or combinations of metals) it is typical to list the
temperature at which the metal alloy first begins to melt (the solidus) and
the temperature at which the last bit of metal finally dissolves into the
liquid phase (the liquidus). You can also understand that if the
manufacturer of a metal alloy has a little bit of variation when he makes
up each batch of alloy, there may be some variation in liquidus and solidus
temperatures. Because in our specific phase diagram the solidus line is a
straight horizontal line at 779.1oC, varying the composition of this
particular alloy will not change the solidus temperature, only the liquidus
temperature.
I hope this helps you understand a rather complex subject. It isn't easy.
I have drawn a vertical line at the 5% Cu composition. This line represents the
composition of sterling silver.
According to this diagram, as a lump of sterling silver is heated, nothing
much happens until the temperature reaches about 760oC or so. Above that
temperature, the sterling silver starts to melt. The phase diagram shows
us that the composition of the material that first melts contains 28.1%
copper (and 71.9% silver), while that portion of the lump which hasn't
melted is essentially pure silver. As the mixture is heated to about
800oC, the green line shows that the liquid portion of the mixture now has
a composition of roughly 20% copper, with the solid phase still consisting
of essentially pure silver. At 900oC, the liquid phase contains about 8%
copper (as more and more silver has dissolved into the liquid phase) but
still contains some solid silver. Not until the temperature reaches 930 or
940oC has all of the silver dissolved into the liquid phase.
In metal alloys (or combinations of metals) it is typical to list the
temperature at which the metal alloy first begins to melt (the solidus) and
the temperature at which the last bit of metal finally dissolves into the
liquid phase (the liquidus). You can also understand that if the
manufacturer of a metal alloy has a little bit of variation when he makes
up each batch of alloy, there may be some variation in liquidus and solidus
temperatures. Because in our specific phase diagram the solidus line is a
straight horizontal line at 779.1oC, varying the composition of this
particular alloy will not change the solidus temperature, only the liquidus
temperature.
I hope this helps you understand a rather complex subject. It isn't easy.
Current Queue |
Current Queue for Chemistry |
Chemistry archives
Try the links in the MadSci Library for more information on Chemistry.
MadSci Home | Information |
Search |
Random Knowledge Generator |
MadSci Archives |
Mad Library | MAD Labs |
MAD FAQs |
Ask a ? |
Join Us! |
Help Support MadSci
MadSci Network,
webadmin@www.madsci.org
© 1995-2001. All rights reserved.
 I have drawn a vertical line at the 5% Cu composition. This line represents the
composition of sterling silver.
According to this diagram, as a lump of sterling silver is heated, nothing
much happens until the temperature reaches about 760oC or so. Above that
temperature, the sterling silver starts to melt. The phase diagram shows
us that the composition of the material that first melts contains 28.1%
copper (and 71.9% silver), while that portion of the lump which hasn't
melted is essentially pure silver. As the mixture is heated to about
800oC, the green line shows that the liquid portion of the mixture now has
a composition of roughly 20% copper, with the solid phase still consisting
of essentially pure silver. At 900oC, the liquid phase contains about 8%
copper (as more and more silver has dissolved into the liquid phase) but
still contains some solid silver. Not until the temperature reaches 930 or
940oC has all of the silver dissolved into the liquid phase.
In metal alloys (or combinations of metals) it is typical to list the
temperature at which the metal alloy first begins to melt (the solidus) and
the temperature at which the last bit of metal finally dissolves into the
liquid phase (the liquidus). You can also understand that if the
manufacturer of a metal alloy has a little bit of variation when he makes
up each batch of alloy, there may be some variation in liquidus and solidus
temperatures. Because in our specific phase diagram the solidus line is a
straight horizontal line at 779.1oC, varying the composition of this
particular alloy will not change the solidus temperature, only the liquidus
temperature.
I hope this helps you understand a rather complex subject. It isn't easy.
I have drawn a vertical line at the 5% Cu composition. This line represents the
composition of sterling silver.
According to this diagram, as a lump of sterling silver is heated, nothing
much happens until the temperature reaches about 760oC or so. Above that
temperature, the sterling silver starts to melt. The phase diagram shows
us that the composition of the material that first melts contains 28.1%
copper (and 71.9% silver), while that portion of the lump which hasn't
melted is essentially pure silver. As the mixture is heated to about
800oC, the green line shows that the liquid portion of the mixture now has
a composition of roughly 20% copper, with the solid phase still consisting
of essentially pure silver. At 900oC, the liquid phase contains about 8%
copper (as more and more silver has dissolved into the liquid phase) but
still contains some solid silver. Not until the temperature reaches 930 or
940oC has all of the silver dissolved into the liquid phase.
In metal alloys (or combinations of metals) it is typical to list the
temperature at which the metal alloy first begins to melt (the solidus) and
the temperature at which the last bit of metal finally dissolves into the
liquid phase (the liquidus). You can also understand that if the
manufacturer of a metal alloy has a little bit of variation when he makes
up each batch of alloy, there may be some variation in liquidus and solidus
temperatures. Because in our specific phase diagram the solidus line is a
straight horizontal line at 779.1oC, varying the composition of this
particular alloy will not change the solidus temperature, only the liquidus
temperature.
I hope this helps you understand a rather complex subject. It isn't easy.