As usual in chemistry, we are explaining an experimental fact by using an explanatory model. The reasoning, however, has to be based on a more sophisticated model than the Lewis structures we usually use. I will present the three common models in turn, below, and you may stop when you reach an explanation that's too complicated!
From a Lewis point of view, there is no reason why carbon can't form a quadruple bond:
This model satisfies the Octet Rule and leaves no electrons for further bonding. But it implies that C2 is a perfectly stable molecule, like N2, and that just isn't the case. C2 is highly reactive and can only be studied under high-vacuum conditions, when it can avoid meeting other molecules for long enough to be detected.
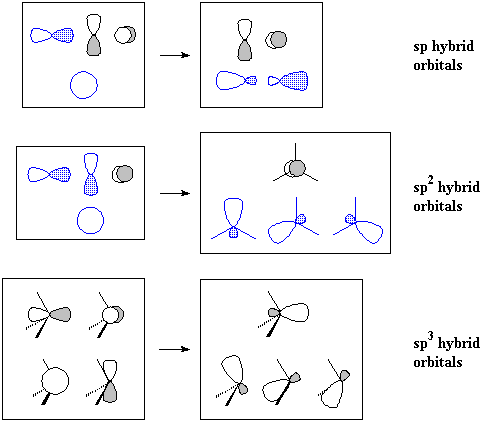
If we go on to the valence-bond model, in which bonds result from the overlap of atomic orbitals, we see a better explanation: carbon cannot form a quadruple bond because it doesn't have enough atomic orbitals pointing in the right directions. Furthermore, the s atomic orbitals must be hybridized (as sp3, sp2 or sp hybrids) so that they may point in some direction.
|
|
| |
Notice that sp hybridization leaves two p orbitals over, while sp2 hybridization leaves one p orbital.
Transition metals are able to form quadruple bonds because they can involve d atomic orbitals in bonding. A quadruple bond requires one s bond, two p bonds and a d bond (between two unhybridized d orbitals).
Valence-bond theory predicts two possible bonding states for C2: a double bond with all electrons paired, and a triple bond with two unpaired electrons. These are called resonance structures, and both must be considered as partial representations of the real situation. The Lewis representations are shown below. For the orbital overlap picture, compare that in carbon monoxide.
Finally, we can visit the molecular-orbital model. Although this model is not required to explain why carbon can't form quadruple bonds, it is required to explain why C2 actually forms only a double bond (!!) and has a triplet ground spectroscopic state (3Pu), in which two of the electrons are unpaired. Valence-bond theory predicts either an all-electrons-paired double bond, or a two-unpaired-electrons triple bond, but not both!
To understand this, we need to recognize that
- in molecular-orbital theory, molecular orbitals are formed from the
overlap of atomic orbitals before they are occupied. s orbitals are formed by the combination
of two atomic s orbitals, or by two p orbitals laid end-to-end; p orbitals are formed by the combination
of two atomic p orbitals laid side-by-side.
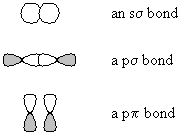
- Furthermore, each pair of atomic orbitals produces one bonding molecular orbital and one antibonding molecular orbital; if bonding and antibonding molecular orbitals are occupied, there is no net bond.
- Finally, while electrons normally pair up in molecular orbitals, it takes energy to make them occupy the same orbital ("correlation energy") and sometimes it takes less energy to put them in different orbitals than it does to put them in the same one.
Homonuclear diatomic molecules (that is, molecules with two atoms only, and those of the same element) have the following molecular orbitals.
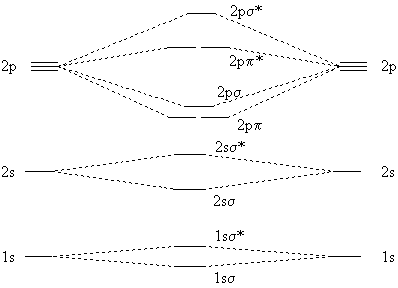
|
Two carbon atoms have a total of 12 (6+6) electrons; when we put 12 electrons into the molecular orbital diagram we at first expect all electrons to be paired:
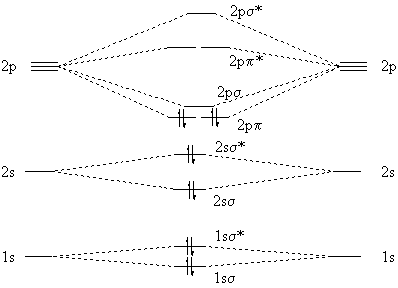
|
However, the experimental fact is that C2 has a 3Pu ground state, so two electrons must be unpaired. We explain this by noting that the 2pp and 2ps molecular orbitals are quite close in energy, so it takes more energy to pair the electrons than to put one in a slightly higher orbital. The resulting molecular-orbital model of C2 is
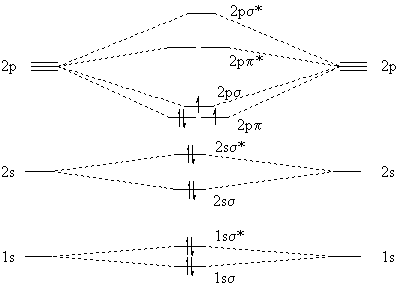
|
Notice that both molecular-orbital models show a net double bond between the carbon atoms.
To summarize:
- To answer your question, we have to go beyond Lewis structures.
- Valence-bond theory answers your question by picturing C2 as a pair of resonance structures,
neither of which have a quadruple bond:
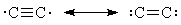
- To explain all the spectroscopic facts about C2, we have to go to a molecular-orbital model, which depicts C2 as rather like O2: only a double bond, but two unpaired electrons.
References:
- Pauling, Linus. The Nature of the Chemical Bond
- Atkins, P.W. Molecular Quantum Mechanics
| Dan Berger |
| Bluffton College |
| http://cs.bluffton.edu/~berger |