The answer is "yes and no." There are no compounds in which a carbon atomic ion exists (it would have a -4 or +4 charge!). But there are in fact compounds in which a carbon is pretty much ionic, while remaining covalently bonded to other atoms. These compounds are often considered to be "almost covalent." Let me explain.
There is no such thing as a pure ionic bond. All bonds involve the sharing of electrons to some degree, however small. You may have been taught that a difference in electronegativity of so much (usually greater than 2 Pauling units) is an ionic bond; anything less than that is a covalent bond. But of course, you can have any value of electronegativity difference, values just over 2 and values just under 2. What makes a compound ionic? The electronegativity difference between sodium and sulfur is 1.65 Pauling units; that between silicon and fluorine is 2.08 Pauling units. So we'd expect sodium sulfide (Na2S) to be covalent and silicon tetrafluoride (SiF4) to be ionic, right? Sorry!
We tend to define ionic bonds operationally, by the way a particular compound behaves under certain conditions. For example, does the compound form a regular ionic crystal structure like sodium sulfide, or does it form separate molecules like silicon tetrafluoride? Is the melting point high (Na2S, 1180°) or low (SiF4, -90.2°)? What about the boiling point (Na2S, not even reported; SiF4, -86°)?
Now that I've said all that, let's talk about ionic organic compounds.
There are organometallic compounds which have bonds between carbon and the alkali metals: alkyllithium and -sodium compounds. The electronegativity differences involved are 1.57 and 1.68 respectively, similar to Na2S. They behave chemically as if they were "carbanions" (carbon anions). But they do not ionize in ether solution -- you can't dissolve them in water; you get an explosion if you try! -- even though you can get separated ions of sodium chloride in ether. Organoalkali compounds do not crystallize in regular ionic-type lattices, but instead in tetrameric forms that show distinctly covalent interactions between the carbon and alkali metal atoms (alkyllithiums preserve this tetrameric structure in solution, while alkylsodiums are hexameric). But as you go down the alkali metals toward cesium, it is reported that the behavior of the carbon-metal bond becomes more and more ionic.
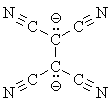 |
 |
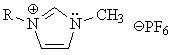 |
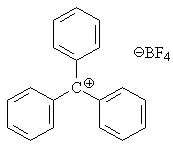
You can also get ionic or nearly ionic compounds with cationic carbon, as long as the anion doesn't have a strong affinity for bonding to carbon and the carbocation is supported by electron-donating groups. For example, triphenylmethyl tetrafluoroborate, (C6H5)3C+ BF4-, is unquestionably ionic.
Finally, there are now a number of organic liquids that are ionic, true molten salts at room temperature. They are being marketed as industrial solvents because they are easy to recover, they dissolve lots of different substances and they are completely non-volatile. But in many or most of them the carbon atoms cannot be said to be ionized.
References used include Greenwood and Earnshaw, Chemistry of the Elements, 1st Ed. (Pergamon Press, 1984) and the CRC Handbook of Chemistry and Physics, 61st Ed. (1980).
| Dan Berger |
| Bluffton College |
| http://cs.bluffton.edu/~berger |