Date: Thu Sep 16 14:34:17 1999
Posted By: Nigel Barker, Head of Science, International School of Lusaka
Area of science: Chemistry
ID: 937355721.Ch
Message:
I agree with your teacher - I don't even know the diagonal rule myself,
although I have seen some of my students using it.
Here's what you do:
You remember that the number of sub-levels is the same as the main energy
level (principal quantum number, if you call it that).
So the first level has one sub-level, the second has two, and so on.
The names of the sub-levels is another thing you have to remember:
| main level | sub-levels present |
| 1 | s |
| 2 | s,p |
| 3 | s,p,d |
| 4 | s,p,d,f |
etc (sub-levels beyond f are always empty in the ground state)
The number of the main level corresponds to the period number in the
periodic table. First period, first main energy level; period 2, main level
2, etc.
Its also worth remembering the labelling of the 'blocks' of the periodic
table:
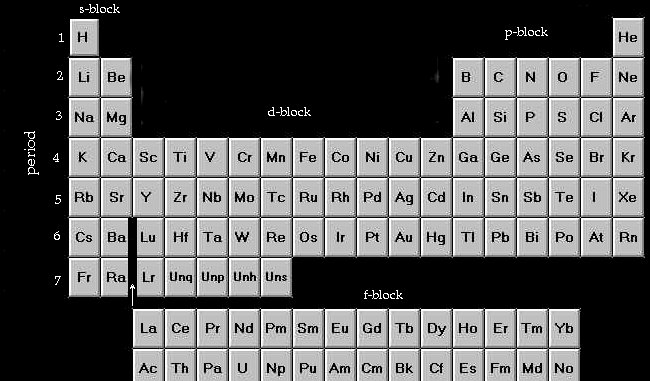 Thats it for things to remember (almost). From now on you can use the
periodic table to work out the rest.
Look at period 1. There are two elements. You remember that there is only
one sublevel, and you also remember that it is called 's'. How many
electrons can go in the s sub-level?
Thats it for things to remember (almost). From now on you can use the
periodic table to work out the rest.
Look at period 1. There are two elements. You remember that there is only
one sublevel, and you also remember that it is called 's'. How many
electrons can go in the s sub-level?
Two - one for H and two for He
The electron configurations are 1s1 for H and 1s2 for
He.
Each element has the electron configuration of the previous element +1
electron.
Now period 2. There are 8 elements. The first two are in the s-block, so
their
configurations are
Li: 1s2 2s1
Be: 1s2 2s2
The next bunch are in the p-block, and there are 6 of them. That means the
p sub-level can hold up to 6 electrons
B: 1s2 2s22p1
C: 1s2 2s22p2
....
Ne 1s2 2s22p6
Now comes the final thing to remember. Period 3 is level 3, which has three
sub-levels (s,p,d). However, if you look at period 3, there are no d-block
elements there. The 3 d sub-level is in the row below (period 4). So the
thing to remember is that the d-block has been shoved down a row. 3d is in
period 4, 4d is in period 5, 5d is in period 6, etc.
Some examples from period 3:
Mg: 1s2 2s22p63s2
Al: 1s2
2s22p63s23p1
Ar: 1s2 2s22p63s23p6
Now period 4:
K: 1s2
2s22p63s23p64s1
Sc: 1s2
2s22p63s23p64s23d1
Zn: 1s2
2s22p63s23p64s23d10
Br: 1s2
2s22p63s23p64s23d104p5
notice that the d-block has 10 columns - the d sub-level takes up to 10
electrons.
when you get down to period 6, you see that there is a gap where the
f-block is supposed to be. A quick glance at the table above shows that the first level with an f sub-level is
4, so its 4f that is being filled up from La to Yb, and 5 f for the
actinides.
To double check that you've got it right, count the total number of
electrons in your configuration. It has to be the same as the atomic number
of the element.
Thats it!
Current Queue |
Current Queue for Chemistry |
Chemistry archives
Try the links in the MadSci Library for more information on Chemistry.
MadSci Home | Information |
Search |
Random Knowledge Generator |
MadSci Archives |
Mad Library | MAD Labs |
MAD FAQs |
Ask a ? |
Join Us! |
Help Support MadSci
MadSci Network,
webadmin@www.madsci.org
© 1995-1999. All rights reserved.
 Thats it for things to remember (almost). From now on you can use the
periodic table to work out the rest.
Look at period 1. There are two elements. You remember that there is only
one sublevel, and you also remember that it is called 's'. How many
electrons can go in the s sub-level?
Thats it for things to remember (almost). From now on you can use the
periodic table to work out the rest.
Look at period 1. There are two elements. You remember that there is only
one sublevel, and you also remember that it is called 's'. How many
electrons can go in the s sub-level?