Date: Sun Sep 26 07:58:52 1999
Posted By: Yaxun Liu, Grad student, Electrical Engineering, National University of Singapore
Area of science: Physics
ID: 938102151.Ph
Message:
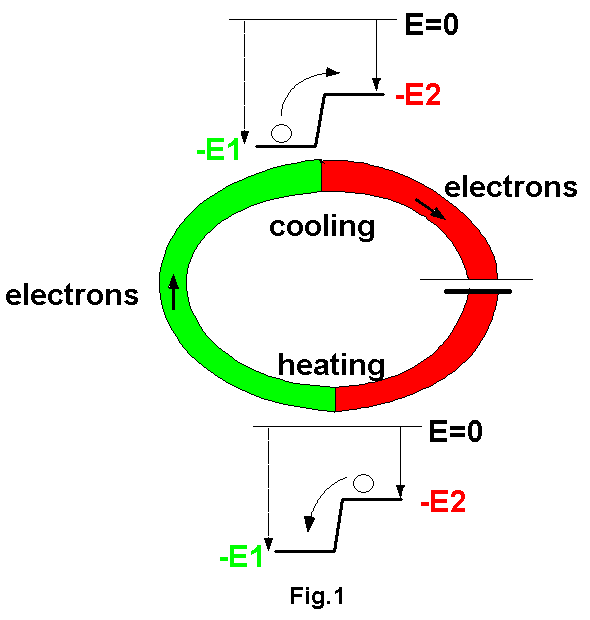 Fig.1
shows two metal strips and a battery connected together. We know that metal
has some "binding" forces on the conductive electrons moving in it. When
we pull out a electron from a metal we need to do some work, therefore
a electron in a metal has some negative potential energy compared to a
free electron. (If that seems difficult to understand, imagine a ball.
If a ball is at a higher position, it has some positive energy because
when it falls, this energy will convert to its kinetic energy. However,
if it is at a lower position, we need to do some work to bring it up to
our level, therefore it has a negative potential energy when it is at a
lower position.) In fig.1 the "green metal" has a lower energy level than
the "red metal". At the upper junction, when a electron moves from the
green metal into the red metal, part of its kinetic energy will convert
to potential energy and it will move slower. Since the temperature is just
a representation of the average speed of the random movements of the
particles,
the temperature at the upper junction seems to decrease. Of course at the
upper junction there are also electrons moving back from the red into the
green, but a electric current means a net electron flux from the green
to the red, therefore a net cooling effect. Similarly, At the lower
junction,
there is a net flux of electrons from the red to the green, therefore a
net heating effect.
Fig.1
shows two metal strips and a battery connected together. We know that metal
has some "binding" forces on the conductive electrons moving in it. When
we pull out a electron from a metal we need to do some work, therefore
a electron in a metal has some negative potential energy compared to a
free electron. (If that seems difficult to understand, imagine a ball.
If a ball is at a higher position, it has some positive energy because
when it falls, this energy will convert to its kinetic energy. However,
if it is at a lower position, we need to do some work to bring it up to
our level, therefore it has a negative potential energy when it is at a
lower position.) In fig.1 the "green metal" has a lower energy level than
the "red metal". At the upper junction, when a electron moves from the
green metal into the red metal, part of its kinetic energy will convert
to potential energy and it will move slower. Since the temperature is just
a representation of the average speed of the random movements of the
particles,
the temperature at the upper junction seems to decrease. Of course at the
upper junction there are also electrons moving back from the red into the
green, but a electric current means a net electron flux from the green
to the red, therefore a net cooling effect. Similarly, At the lower
junction,
there is a net flux of electrons from the red to the green, therefore a
net heating effect.
This explanation is rather crude. There is a very good article on the
web about the Peltier effect in semiconductors [1], it can also help you
understand the Peltier effect at the junction of two different metals.
References
[1]http://jchemed.chem.wisc.edu/Journal/Issues/1996/Oct/abs940.html.
Current Queue |
Current Queue for Physics |
Physics archives
Try the links in the MadSci Library for more information on Physics.
MadSci Home | Information |
Search |
Random Knowledge Generator |
MadSci Archives |
Mad Library | MAD Labs |
MAD FAQs |
Ask a ? |
Join Us! |
Help Support MadSci
MadSci Network,
webadmin@www.madsci.org
© 1995-1999. All rights reserved.
 Fig.1
shows two metal strips and a battery connected together. We know that metal
has some "binding" forces on the conductive electrons moving in it. When
we pull out a electron from a metal we need to do some work, therefore
a electron in a metal has some negative potential energy compared to a
free electron. (If that seems difficult to understand, imagine a ball.
If a ball is at a higher position, it has some positive energy because
when it falls, this energy will convert to its kinetic energy. However,
if it is at a lower position, we need to do some work to bring it up to
our level, therefore it has a negative potential energy when it is at a
lower position.) In fig.1 the "green metal" has a lower energy level than
the "red metal". At the upper junction, when a electron moves from the
green metal into the red metal, part of its kinetic energy will convert
to potential energy and it will move slower. Since the temperature is just
a representation of the average speed of the random movements of the
particles,
the temperature at the upper junction seems to decrease. Of course at the
upper junction there are also electrons moving back from the red into the
green, but a electric current means a net electron flux from the green
to the red, therefore a net cooling effect. Similarly, At the lower
junction,
there is a net flux of electrons from the red to the green, therefore a
net heating effect.
Fig.1
shows two metal strips and a battery connected together. We know that metal
has some "binding" forces on the conductive electrons moving in it. When
we pull out a electron from a metal we need to do some work, therefore
a electron in a metal has some negative potential energy compared to a
free electron. (If that seems difficult to understand, imagine a ball.
If a ball is at a higher position, it has some positive energy because
when it falls, this energy will convert to its kinetic energy. However,
if it is at a lower position, we need to do some work to bring it up to
our level, therefore it has a negative potential energy when it is at a
lower position.) In fig.1 the "green metal" has a lower energy level than
the "red metal". At the upper junction, when a electron moves from the
green metal into the red metal, part of its kinetic energy will convert
to potential energy and it will move slower. Since the temperature is just
a representation of the average speed of the random movements of the
particles,
the temperature at the upper junction seems to decrease. Of course at the
upper junction there are also electrons moving back from the red into the
green, but a electric current means a net electron flux from the green
to the red, therefore a net cooling effect. Similarly, At the lower
junction,
there is a net flux of electrons from the red to the green, therefore a
net heating effect.