| MadSci Network: Chemistry |
Mr. Scully: Please bear with me while I address those non-chemists who may be visiting this answer! I will address your specific concerns at the end.
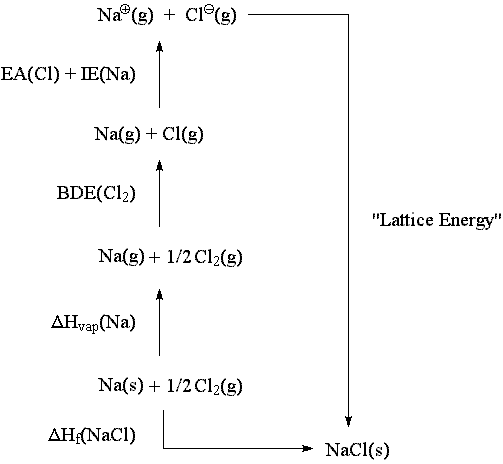
All of what you have stated is absolutely correct. The correct thermodynamic sequence for conversion of sodium and chlorine (in their standard states) to sodium chloride (in its standard state) is shown here:

The values of the thermodynamic quantities shown are taken from the NIST Chemistry WebBook, and are as follows:
Therefore, it is certainly true that the bald statement explaining "bonding as a phenonemon that occurs so that atoms can fill their valence shells" is a drastic oversimplification. The actual reason for most chemical reactions is that the energy of the resulting system is lower than the energy of the starting materials. Spontaneous reactions are exergonic: they involve a lowering of overall free energy.
Nevertheless, "filling the valence shell," and the Lewis model in general, give students a powerful conceptual tool, which not only explains chemical bonding -- especially covalent bonding -- in an easy-to-understand, though approximate, way, but also explains trends in the electron affinities and ionization energies of the elements (see another of my MadSci answers). Sure it's approximate, but you can't talk about chemical bonding in anything except H2+ without using approximations!
The key here is that, by stressing the filling of valence shells and the Lewis picture of bonding, we are substituting an easy-to-understand, useful approximation for a more-difficult, harder-to-explain approximation. I would expect that your students might like to be shown the role of lattice energy in the formation of ionic compounds (although if you can calculate all the coulomb interactions* from first principles you're a better mathematician than I am). I know from experience that students do not prefer a comprehensive course in molecular orbital theory -- which is also an approximation! -- to the Lewis picture.
| Dan Berger | |
| Bluffton College | |
| http://cs.bluffton.edu/~berger |
| * "Lattice Energy" is composed mostly of nearest-neighbor coulombic attractions which may easily be calculated -- with an additional component from non-nearest-neighbor attractions and repulsions which is much harder to calculate but may be found by comparing the calculated nearest-neighbor attraction energy with the actual energy calculated, from the thermodynamic cycle, for conversion from gas-phase ions to the ionic solid. |
Try the links in the MadSci Library for more information on Chemistry.