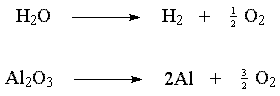
| MadSci Network: Chemistry |
What are some new
practical applications of electrolysis?
For my AP Chemistry class, my group and I have to try
and find some practical applications for electrolysis (not including
hair removal) and we were wondering if you could give us a hand. We
are also interested in the theory of electrolysis and how it works.
In chemistry, electrolysis refers to any process in which a chemical reaction is driven by electricity. The general name for the field is electrochemistry. Examples include splitting water into hydrogen and oxygen or smelting bauxite (aluminum oxide) by splitting it into aluminum and oxygen.
Electrochemical processes are uniformly based on redox (reduction/oxidation) reactions. Think of it as the opposite of a battery. In a battery the chemical process is spontaneous and produces electrical energy, while in an electrolysis you need to put electrical energy in to run the chemical process.
An electrolytic cell works on the same principles as any other electrical device: electrons are stripped away from the anode and transferred to the cathode. In an electrolytic reaction, the desired substance appears at one or the other electrode; which electrode depends on whether the substance is being oxidized or reduced.
For example, in the electrolysis of water hydrogen is being reduced and bubbles out at the cathode; oxygen is being oxidized and collects at the anode. (This sounds strange, but if you remember that electrolysis is a reversal of burning -- or oxidizing -- hydrogen, it will make sense.)
Electrochemistry is used in the synthesis of organic molecules. Often electrochemical reactions can proceed under milder conditions (more safely), with less hazardous waste (better for the environment), and in higher yields (better for your wallet) than a similar process using chemical reagents. Some synthetic processes can only be done using electrochemical methods, and electrochemical methods are used in the large-to-medium-scale synthesis of organic "fine chemicals" because of the financial, safety and environmental advantages. Here's a page outlining some current research by one of my graduate school professors.
I can think of at least three common uses of electrochemistry:
| Dan Berger | |
| Bluffton College | |
| http://cs.bluffton.edu/~berger |
Try the links in the MadSci Library for more information on Chemistry.