Dipole moment is due to the degree of charge separation in a molecule. How it is calculated (or how the degree of charge separation can be determined from a dipole moment, since that's what's obervable) is given in most physics textbooks, but normally it's a product of the quantity of charge and the distance between positive and negative.
All polar molecules have per manent dipole moments, meaning that they have a positive end (usually carbon or hydrogen) and a negative end (usually oxygen, nitrogen or a halogen). "Temporary" dipole moments are discussed here.
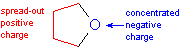
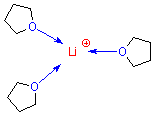
But some polar molecules are also good at coordinating highly-charged species, such as ions. For example, ethers are good at coordinating alkali metal cations. This is because negative charge is fairly concentrated on the oxygen atom of the ether. But ethers are not good at coordinating halide anions! This is because the positive charge in ethers is widely spread among several different carbon and hydrogen atoms and doesn't present a "concentrated target" for an anion.
 |
 |
| charge
separation in an ether molecule |
ether
molecules coordinating a lithium cation |
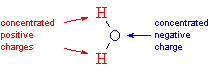
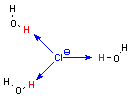
So-called protic solvents all contain hydrogen atoms bonded directly to electronegative atoms (oxygen or nitrogen). Since the hydrogen atom is electropositive, it takes on a positive charge; because the hydrogen atom is very small, the positive charge is quite concentrated. This means that protic solvents are good at coordinating both cations and anions, because they have both a concentrated negative charge (the oxygen or nitrogen atom) and a concentrated positive charge (the hydrogen bonded to oxygen or nitrogen).
 |
 |
| charge
separation in a water molecule |
water
molecules coordinating a chloride anion |
The center of negative charge on one molecule can also coordinate the center of positive charge on a neighboring molecule, if it is concentrated enough. Because a concentrated yet non-ionic center of positive charge is always a hydrogen atom, this is called a hydrogen bond. Hydrogen bonds give ice its open structure and hold together the double-helix of DNA.
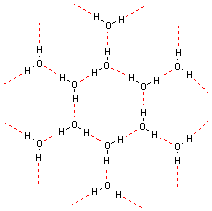 |
| Hydrogen bonding in an ice crystal |
| Dan Berger |
| Bluffton College |
| http://cs.bluffton.edu/~berger |