There are quite a number of common definitions, but most of them are too restrictive and have exceptions. Incidentally there's a very good answer on the Web, to the question of "what is organic chemistry?"
Organic compounds are produced by living things. Inorganic compounds are produced by non-living natural processes or by human intervention in the laboratory.
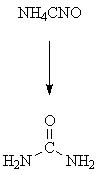 This was the most common definition of "organic" until
Wohler's 1828 synthesis of urea (an organic compound) from
ammonium cyanate (a salt, and ┐therefore? inorganic). But we no
longer use this definition, for the simple reason that many compounds that
everyone agrees are organic -- including "natural products" which
are routinely made by living things -- have been synthesized by humans.
Some of these natural products are synthesized by the ton. And
unquestionably organic molecules, such as the amino acid glycine, have been
found in
interstellar space where there are no living things.
This was the most common definition of "organic" until
Wohler's 1828 synthesis of urea (an organic compound) from
ammonium cyanate (a salt, and ┐therefore? inorganic). But we no
longer use this definition, for the simple reason that many compounds that
everyone agrees are organic -- including "natural products" which
are routinely made by living things -- have been synthesized by humans.
Some of these natural products are synthesized by the ton. And
unquestionably organic molecules, such as the amino acid glycine, have been
found in
interstellar space where there are no living things.
Some people apparently still think this definition should hold, but it's just not so.
Inorganic compounds can form salts. Organic compounds can't.
This definition seems to work as a practical matter under very limited circumstances. For example, it allows us to distinguish between the organic and inorganic cyano group, which have rather different chemical behaviors in e.g. acetonitrile and sodium cyanide.But this definition is far too limited. Not only are there plenty of inorganic compounds that don't form salts (for example, sulfur hexafluoride SF6), but there are a number of organic compounds that do form salts: not only carboxylic acids but amines, alcohols, and even acetylene can form salts. And how do we distinguish between (organic) sodium acetylide, NaC║CH, and (inorganic) calcium carbide, CaC║C?
Some chemists would call lithium acetylide, potassium acetate and sodium methoxide ("sodium methylate") inorganic, while calling acetylene, acetic acid and methanol (from which the salts are derived) organic -- but I'm not one of them.
Furthermore there are now a number of indubitably organic compounds that are nevertheless stable salts. For more, see this answer.
Organic compounds contain carbon. Inorganic compounds don't.
This definition is often given but is no help at all. What do we make of carbon dioxide, sodium cyanide, baking soda (sodium bicarbonate), ...?Organic compounds contain carbon-hydrogen bonds. Inorganic compounds don't.
This is a much better definition, allowing us to call sodium acetylide "organic" but calcium carbide "inorganic," but it doesn't always work. We can certainly distinguish the compounds listed above as inorganic by this rule, but what about tetracyanoethylene, which is indubitably organic but contains no hydrogen atoms at all?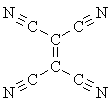
tetracyanoethylene 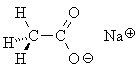
sodium acetate Nevertheless, almost all "organic carbon" has carbon-hydrogen bonds, while all "inorganic carbon" does not have carbon-hydrogen bonds. Even if you call e.g. sodium acetate inorganic, everyone will agree that the carbon atom which is attached to the hydrogens is "organic carbon."
Inorganic compounds contain metal atoms. Organic compounds don't.
This doesn't really work any too well either. Even leaving the huge field of organometallic chemistry out of the running, are we really going to call soap (sodium salts of fatty acids) or the lipid bilayers forming cell membranes (again, salts of long-chain organic acids) "inorganic"???An organic compound is whatever an organic chemist says it is; an inorganic compound is whatever an inorganic chemist says it is.
In practice the distinction often reduces to this. If an organic chemist is studying it, it's probably an organic compound. If an inorganic chemist is studying it, these days it's still probably an organic compound... many inorganic chemists are busily studying the biological uses of metal atoms and so forth, and in biological systems metals are firmly incorporated into an organic chemical matrix!Of course that's not entirely fair. Normally organic chemists study organic or organometallic compounds, and inorganic chemists study organometallic compounds or inorganic compounds. Organometallic chemistry is where the fields meet, and it's neither "organic" nor "inorganic" any more than biochemistry is exclusively either "biology" or "chemistry."
| Dan Berger |
| Bluffton College |
| http://cs.bluffton.edu/~berger |